Biology Reference
In-Depth Information
(B)
(A)
rs2004640
P = 0.0006
rs4728142
P = 0.0023
80
70
60
80
70
60
50
40
30
20
10
0
50
40
30
20
10
0
GG/GT
TT
GG/GA
AA
Genotype
Genotype
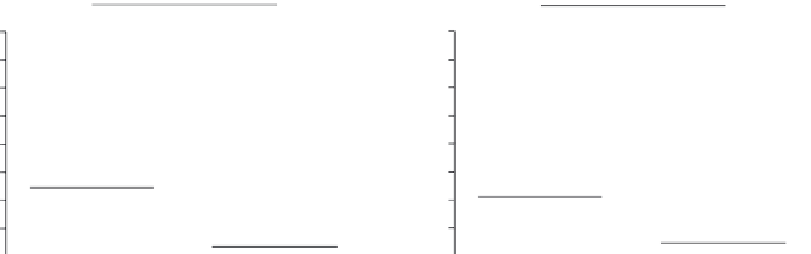
FIGURE 3.8
Relationship between
IRF5
genotypes and pharmacological response to IFN-β treatment.
Pharmacological response at 1 month following the start of IFN-β treatment was determined in a cohort of
30 patients with RRMS and the association with rs2004640 (A) and rs4728142 (B) genotypes was determined.
Pharmacological responses are lower in patients homozygous for the rs2004640 T allele (A) or rs472814 A-allele
(B) compared to other genotypes.
(Adapted with permission from: Vosslamber S; van der Voort LF; van den Elskamp IJ;
Heijmans R; Aubin C; Uitdehaag BM, et al. Interferon regulatory factor 5 gene variants and pharmacological and clinical out-
come of Interferon beta therapy in multiple sclerosis. Genes Immun. 2011; 12: 466-72.)
Fig. 3.8
)
[53]
. Moreover, patients with the rs2004640-TT genotype developed more magnetic
resonance imaging (MRI)-based T2 lesions during IFN-β treatment (p = 0.003). Accordingly, an
association between MRI-based non-responder status and rs2004640-TT genotype was observed
(p = 0.010). The clinical relevance of the rs2004640-TT genotype was validated in an independ-
ent cohort wherein a shorter time to first relapse was found (p = 0.037). These findings suggest
a role for IRF5 gene variation in the pharmacological and clinical outcome of IFN-β therapy that
might have relevance as a biomarker to predict the response to IFN-β in RRMS.
Altogether the above findings indicate that the presence of the type I IFN signature
defines two clinically distinct subsets of MS patients, based on the clinical response outcome
of IFN-β therapy. The IFN
high
patients represent a group of patients who express a failure to
demonstrate a pharmacological response to IFN-β treatment and consequently do not show
a clinical response. IFN
low
patients exert a pharmacological response with concomitant clin-
ical response. The published results warrant further studies to validate the clinical utility
of the IFN signature as biomarker to predict the response to IFN-β treatment. Due to the
temporal aspects related to monitoring of the clinical response, it remains to be determined
whether research findings from studies on the primary phase of unresponsiveness might be
intimately linked to processes that are (also) related to NAb development.
Acknowledgments
Supported in part by the Center for Medical Systems Biology (a center of excellence approved by the Netherlands
Genomics Initiative
/
Netherlands Organization for Scientific Research), the Center for Translational and Molecular
Medicine (CTMM) (an initiative from the ministry of Economic Affairs of The Netherlands) and grants from the
Dutch Arthritis Foundation and MS Research (04-549 MS and 08-660 MS).







































Search WWH ::

Custom Search