Biology Reference
In-Depth Information
P = 0.0052
Good responders
Non-responders
350
70
350
40
IFN
CD19 count
IFN
CD19 count
60
300
35
300
30
250
50
250
25
200
40
200
20
150
30
150
15
100
100
20
10
50
10
50
5
0
0
0
0
t=0
t=3
t=6
t=0
t=3
t=6
FIGURE 3.6
Pharmacodynamics of the interferon (IFN) type I-response activity during rituximab treatment
reveal marked differences between responders and non-responders. Shown are pharmacodynamic measure-
ments in 13 patients with rheumatoid arthritis (RA) of a set of six type I IFN response genes (IFI44, IFI44L, HERC5,
RSAD2, LY6E, and Mx1) (left y axis) and B cell counts based on CD19 cytometry (right y axis) at baseline (t0), 3 (t3)
and 6 months (t6). Patients were stratified in responders and non-responders based on changes in disease activity
score (ΔDAS) criteria. Pharmacodynamic analyses of the IFN type I-response activity during rituximab treatment
revealed marked differences between responders and non-responders for baseline IFN response activity (ΔDAS
p = 0.0052) and the rituximab induced increase in IFN type I activity at 3 months (ratio t3
/
t0) (ΔDAS p = 0.049).
The change in IFN response gene activity during rituximab treatment negatively correlated with the correspond-
ing baseline level, although no significance was reached (p = 0.0576; R = −0.53).
(Adapted with permission from:
Vosslamber S. et al.
[53]
.)
IFN response activity to a level comparable with that of non-responders during three
months of treatment, whereas non-responders displayed an activated type I IFN-system
already before the start of treatment, which remains stable during treatment. The IFN signa-
ture score returned to baseline values at six months after the start of treatment (
Fig. 3.6
).
Thus a pharmacological increase in IFN response activity during rituximab treatment
is associated with a favorable response and may provide insight into the biological mech-
anism underlying the therapeutic response. The dynamic increase in type I IFN activity
might be a determining factor in the ameliorative effect of B cell depletion therapy in RA,
and might explain the increased BAFF
/
BLyS levels, persistence of pathogenic B cells, and
the change in macrophage function. These findings provide a basis for further study on
the role of the IFN signature as a biomarker for effective dosing and timing of treatment,
towards patient-tailored treatment in RA.
A dynamic increase in IFN response activity with concomitant B cell depletion may be a
prerequisite for a beneficial outcome. This hypothesis may also explain the beneficial effects
of rituximab treatment observed in multiple sclerosis, a disease that responds favorably to
effects of IFN-β. Conversely, a pharmacological increase in the type I IFN activity by rituxi-
mab may lead to disease progression and
/
or an increase in disease activity in type I IFN
driven diseases such as SLE, and may explain the failure to meet clinical endpoints in recent
randomized, placebo-controlled trials of rituximab
[55]
. The data suggest that rituximab











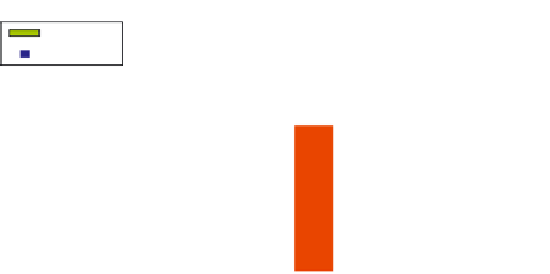














Search WWH ::

Custom Search