Biology Reference
In-Depth Information
in
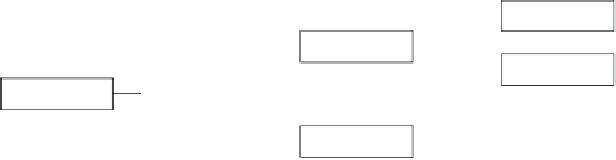
Fig. 7.5
. In this design, all subjects are tested for diagnostic status at baseline and then
randomized into a drug or placebo cohort based on the diagnostic test result. The drawback
of this trial design is that it requires the test to be available at trial start for implementa-
tion. The test must also provide rapid results to ensure timely enrollment and stratification.
In addition, depending on the distribution of negative and positive subjects at screening, it
may slow enrollment if the investigator desires equal distribution in the test positive and
negative groups. In terms of benefits, the trial design allows for the prospective identifica-
tion and testing of the diagnostic hypothesis. The patients are equally distributed among
test negative and positive groups and drug and placebo arms, giving the clearest picture of
diagnostic predictive value. True positive and negative predictive values can be calculated
from this clinical trial design, as clinical response to therapeutic intervention will be avail-
able for both diagnostic negative and positive subjects. This approach also allows the inves-
tigator to understand the performance of the diagnostic in a more commercial setting with
result turnaround times intended to mimic that required by a physician in practice. Based
on the benefits outlined above, this approach is the preferred clinical trial design in the eval-
uation of diagnostic hypotheses. This approach is often used in early and later stage clinical
development where there is a better understanding of diagnostic prevalence and potential
predictive value.
The final clinical trial design for diagnostic assay implementation is the restricted
approach. In this design the trial enrolls only those patients that are diagnostic positive. A
diagram of this clinical trial design is depicted in
Fig. 7.6
.
Drug
Test is
+
Placebo
All PG tested
at
randomization
Drug
All subjects
Test is
−
Placebo
FIGURE 7.5
A diagram of the randomization by diagnostic call clinical design as depicted in the Drug
Diagnostic Co-Development Concept Paper.
Drug
Test is
+
Placebo
All subjects
All tested
Test is
−
FIGURE 7.6
A graphical representation of the restricted clinical design approach as shown in the Drug
Diagnostic Co-Development Concept Paper. Represents an aggressive clinical trial design in which only diagnostic
positive or negative patients are enrolled in a trial.
























Search WWH ::

Custom Search