Biology Reference
In-Depth Information
2.
Clinical Trial Assay - the first stage in migration to clinical applicability
a.
Assay migrated to platform and format applicable to clinical testing environment
b.
Assay components migrated to as many good manufacturing process (GMP) grade
reagents as possible, with avoidance of assay-specific reagents
c.
Assays involved are minimally qualified to establish early performance criteria
d.
Applied to later stage clinical development as an investigational use device
3.
Investigational Usage Only (IUO) Assay - assay further refined for clinical
implementation, represents the final evolution prior to clinically relevant device
a.
Assay is applied to final commercially available platform in appropriate format
b.
Assay components are all GMP grade with no assay-specific reagents
c.
Assays involved are fully validated and evaluated against previously established
performance criteria
d.
Applied in pivotal stage clinical testing to prove hypothesis and provide clinical
validation
4.
IVD - the final commercially available cleared device
a.
Clinically validated device applied to therapeutic product available for physician use
and indicated in therapeutic label.
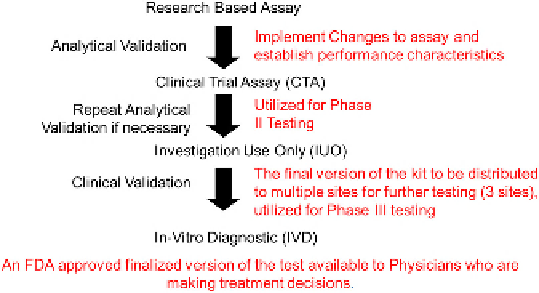
A graphical representation of this progression is given in
Fig. 7.3
. While assays may be in
a hybrid of the stages described here, this general evolution of an assay is followed by the
majority of companion diagnostic devices. As such, it is important to note the stage of the
assay you are developing and make sure it is being applied in the correct stage of develop-
ment with the correct level of analytical and clinical validation. These considerations will be
discussed in greater detail throughout the remainder of the chapter.
7.4.2 General Considerations in Drug Diagnostic Co-Development
Prior to discussing the level of validation and considerations for implementation of poten-
tial diagnostics in clinical development, there are some general concepts of drug diagnostic
FIGURE 7.3
A graphical representation of the natural progression of an assay from discovery through compan-
ion diagnostic with levels of validation necessary included for each step.

Search WWH ::

Custom Search