Biology Reference
In-Depth Information
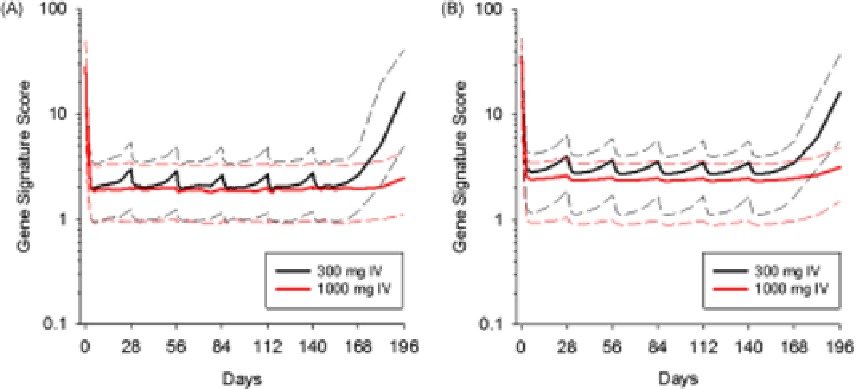
FIGURE 1.9
Simulated type I IFN signature profiles in peripheral blood (A) and skin tissue (B) of SLE patients
upon multiple IV administrations of MEDI-546 (fixed dose) once every four weeks. The solid lines represent the
medians of 1000 simulated profiles while dotted lines represent the lower or upper quartiles. The observed upper
boundaries (mean + 2 standard deviations) of the type I IFN gene signature in the blood and skin of healthy donors
were 2.9 and 1.8, respectively.
Courtesy of Clin. Pharmacol. Ther.
1.4 SAFETY
Adverse drug reactions (ADRs) which are defined by the World Health Organization
(WHO) as: 'harmful, unintended reactions to medicines that occur at doses normally used
for treatment'
[66]
are among the top ten reasons for hospitalization in the United States
[67]
.
Indeed ADRs have resulted in the withdrawal of a number of drugs and multi-billion dollar
lawsuits in recent years. The causes of ADRs and how to prevent them have been the topic
of numerous commentaries
[68]
. Some of the preventable causes of ADRs include medication
errors including wrong medication, inappropriate dose or drug-drug interaction. While the
economic cost of ADRs and how to reduce them is a large subject, in this chapter we focus
on how translational approaches can help to understand the underlying mechanism and how
this understanding in turn can lead to strategies to reduce the likelihood of an ADR.
A common reaction to the question, 'How safe should a drug be?' is typically 'Extremely safe.
There should be no severe ADR.' While this reaction is understandable, no drug is without risk
and in the context of drug development it is more useful to assess the value of any therapeu-
tic to society in terms of its benefit:risk ratio. This ratio is not a constant; rather it can vary by
disease area, and even over time. For example, statins which are used to reduce cholesterol can
be considered to have a high benefit:risk ratio, as they have demonstrated impressive benefit
in reducing cardiovascular morbidity and mortality over two decades and are associated with
very rare ADRs such as rhabdomyolysis with a frequency of one event in 10,000
[69]
. In con-
trast, cancer therapies typically have low response rates and significant ADRs associated with
them, so any new therapeutic that confers incremental benefit over standard of care even when
associated with significant ADRs can have an acceptable benefit:risk ratio. For inflammatory


Search WWH ::

Custom Search