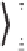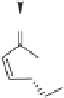Chemistry Reference
In-Depth Information
ether followed by a retro-Diels-Alder reaction afforded the protected hydroxymethyl cy-
clopentenone,
110
. Diastereoselective reduction to the corresponding allyl alcohol and
subsequent palladium-catalyzed substitution afforded an advanced intermediate,
111
, that
was converted into enantiomerically pure (-)-Carbovir and (-)-Abacavir (Scheme 4.29).
(i) Co2(CO)
8
, hexane, rt, 1 h.
O
(ii)
t
Bu
S
PPh
2
O
O
TMS
N
H
H
Toluene,
70 °C, 16 h
.
Bn
TMS
(iii) Norbornadiene, NMO,
CH
2
Cl
2
,70°C,16h.
(53 % overall yield)
H
H
H
OTIPS
(-)-
108
109
CH
2
Cl
2
,55°C,2h
(86 %)
AlMeCl
2
HN
N
N
O
N
N
NH
2
Cl
N
OTIPS
N
OH
N
N
NH
2
(-)-
Abacavir
110
O
OTIPS
N
NH
111
N
N
NH
2
OH
(-)-
Carbovir
Scheme 4.29
Enantioselective approach to various carbanucleosides from chiral cyclopen-
tenone
108
via Pauson-Khand reaction.
In 2009, Shea
et al.
described an interesting study of a tandem intramolecular
Nicholas/PKR strategy for the synthesis of tricyclic oxygen- and nitrogen-containing het-
erocycles.
33
This methodology enables the conversion of simple acyclic starting materials,
112
, into a series of previously unknown heterocycles,
114
. Tricyclic ethers (Z
=
O) with
[5,7,5]- and [5,8,5]-systems and tricyclic [5,7,5]- and [5,8,5]-amine-containing (Z
NTs)
heterocycles were successfully prepared via the Nicholas/PKR reaction (Scheme 4.30) .
=
R
Co(CO)
3
Z
Nicholas
then
Pauson
Khand
m
H
n
(OC)
3
Co
Co
2
(CO)
8
O
m
m
H
Z
OR
OR
Z
H
n
m=1,2
n=1-5
Y=O,NTs
n
112
113
114
Scheme 4.30
Tandem intramolecular Nicholas/Pauson-Khand reaction strategy leading to
tricyclic oxygen- and nitrogen-containing heterocycles.