Chemistry Reference
In-Depth Information
R
R
R
Catalyst
L*(4)
P(4-YC
6
H
4
)
2
P(4-YC
6
H
4
)
2
X
+
X
O
X
O
H
H
69a-e
70a-e
71a-e
dr up to 75:1
ee up to 81 %
(R) BINAPs
72a-d
a
:(Y=H)
b
: (Y = Me)
c
: (Y = OMe)
d
:(Y=CF
3
)
a
: (X = NTs, R = Me)
b
: (X = NTs, R = Ph)
c
: (X = NTs, R = H)
d
: (X = O, R = Me)
e
: (X = O, R =Ph)
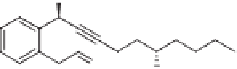
Scheme 4.18
Preparation of vinyl substituted optically active
N
-or
O
-containing bicy-
clo[3.3.0]octenes.
In 2004, Moriarty
et al.
published a general and novel solution to the synthesis of biolog-
ically important stable analogs of prostacyclin PGI2, that is, benzindene prostacyclins.
21
This work provided the first demonstration of the synthetic utility and reliability of the
asymmetric PK cyclization route for the synthesis and subsequent manufacture of com-
plex drug compounds on a multikilogram scale. The key step in the synthesis involves
efficient stereoselection via the PK cyclization of benzoenyne
73
under the agency of
the benzylic OTBDMS group, which serves as a temporary stereodirecting group and is
conveniently removed via benzylic hydrogenolysis concomitantly with the catalytic hy-
drogenation of the enone product (Scheme 4.19). Thus, the benzylic chiral center dictates
the stereochemistry of the stereogenic centers at three carbon atoms, that is, C3a, C9a,
and C1.
H
3
C
O
OTBDMS
Co
2
(CO)
8
(89 %)
OTMDMS
CH
3
CH
2
O
CH
3
O
THP
H
3
C
O
O
THP
73
74
Scheme 4.19
An asymmetric Pauson-Khand cyclization, formation of key intermediate
74
used in the synthesis of prostacyclin PGI
2
analogs.
The PK approach was also successfully applied to the synthesis of three new carbo-
cyclic nucleoside analogs with nucleobases attached to 3
-hydroxymethylcyclopent-2
-en-
1
-yl scaffolds, as reported by Schmalz
et al.
in 2005.
22
A variety of symmetric dienynes
(i.e., propargylic acetals
75
) were used as substrates in the cobalt-mediated PK reac-
tion to yield racemic bicyclic cyclopentenone derivatives
76
with high diastereoselectivity
(Scheme 4.20).