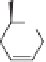Chemistry Reference
In-Depth Information
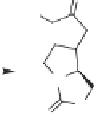
In another approach,
6b
chiral oxazolidinone derivative
27
, which is available in a few
steps from L-glutamic acid, was used as the substrate (Scheme 4.7). In this reaction, the
built-in oxazolidinone ring serves as a rigid template for good diastereofacial selectivity
in the cyclization step, which gives
28
in a 93% yield. Subsequent reduction afforded a
separable mixture of isomers
29
; one isomer was then used to synthesize (-)-kainic acid.
O
O
Me
Me
H
H
H
H
H
(i) Co
2
(CO)
8
(ii) TMANO (93%)
CO
2
H
CO
2
H
H
H
N
N
N
H
O
O
O
O
O
O
27
28
29
(-)-kainic acid
Scheme 4.7
Another aproach to synthesize (-)-kainic acid via the Pauson-Khand reaction.
In the same year, that is, 1994, several studies using carbohydrates as chiral substrates for
PKR appeared. The first attempt at a PKR with carbohydrate derivatives was reported by
Rettie
et al.
; they studied and characterized hexacarbonyldicobalt complexes derived from
2-propynyl and 3-butynyl 4,6-di-O-acetyl-2,3-dideoxy-
-D-erythro-hex-2-enopyranosides
(Scheme 4.8).
8
2,3-Dideoxyhexenepyranosides
30a-d
were prepared from tri-O-acetyl-
D-glucal and an appropriate alcohol in the presence of boron trifluoride etherate. Then,
hex-2-enopyranosides
30a,b
were treated with octacarbonyldicobalt in diethyl ether to
produce hexacarbonyldicobalt complexes
31a,b
as analytically pure red oils in good yields.
However, attempts to promote intramolecular Pauson-Khand reactions of
31a,b
(e.g., in
solution under a CO atmosphere or on SiO
2
) were unsuccessful.
OAc
OAc
OAc
Co
2
(CO)
8
O
O
O
AcO
O
AcO
O
AcO
O
(CH
2
)
n
(CH
2
)
n
(CH
2
)
n
H
O
(OC)
3
Co
Co(CO)
3
R
R
30a
(n = 1)
30b
(n = 2)
30c
(n = 3)
30d
(n = 4)
31a
(n = 1)
31b
(n = 2)
Scheme 4.8
Carbohydrates as chiral substrates in the Pauson-Khand reaction.
Soon after, the first successful PK cyclization of sugar-derived enynes (Scheme 4.9) was
reported by Marco-Contelles
et al al
.
9
The readily available D-glucose-derived 1,6-enyne
precursors,
30
,
32
, and D-galacto derivative,
33
, were converted into the corresponding
cobalt complexes, which readily decomposed upon treatment with NMO to provide bis-
heteroannulated pyranosides
34a-d
and
35
in good yields. The moderate overall yield
of the PKR is compensated for by the efficiency of the one-pot process and the highly
functionalized final products, which are difficult to obtain via other methods.
10