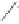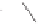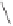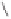Chemistry Reference
In-Depth Information
R
1
Co
4
(CO)
12
(20 mol%)
R
1
( )
( )
n
Triton X-100 (50 mol%)
R
2
n
Z
Z
O
H
2
O, 70 °C, CO(1 atm)
R
2
Z = C(CO
2
Et)
2
, R
1
= Me, R
2
= H (n = 1): 79%
Z = C(C O
2
Et)
2
, R
1
= Ph, R
2
= H (n = 1): 85%
Z = C(CO
2
Me)
2
, R
1
= Me, R
2
= Et (n = 1): 86%
Z = C(CO
2
Et)
2
, R
1
= Me, R
2
= H (n = 2): 76%
Z = CH(CO
2
Et), R
1
= Me, R
2
= H (n = 1): 78%
Z = NTs, R
1
= n-Pr, R
2
= H (n = 1): 84%
Z = O, R
1
= Ph, R
2
= H (n = 1): 70%
Z = CH
2
, R
1
= (CH
2
)
2
SEt, R
2
= H (n = 1): 68%
Scheme 3.32
But a high pressure of CO was needed, substrate scope was narrow, and there was no
comment on the recyclability of the reaction media.
3.9
Intramolecular Reaction of Carbodiimides with Alkynes
Among cumulated compounds, carbodiimide was used in a hetero Pauson-Khand re-
action. Mukai disclosed an intramolecular reaction of
ortho
-phenylene tethered alkyne-
carbodiimide for the synthesis of tricyclic heterocycles.
38
The above mentioned Co
2
(CO)
8
-
TMTU system could operate as a catalyst (Scheme 3.33). The yield was generally
moderate, but this protocol was used as a key reaction for the synthesis of indole alka-
loid (
±
)-physostigmine.
O
R
1
R
1
MeNHCO
Co
2
(CO)
8
(10-20 mol%)
R
2
Me
R
2
TMTU (60-120 mol%)
Benzene, 70 °C
CO (1 atm)
O
R
1
= MeO
R
2
= TMS
R
3
= Me
N•N
N
Me
N
Me
N
N
R
3
H
R
3
(±)-physostigmine
R
1
= H, R
2
= TMS, R
3
= p-MeOC
6
H
4
: 69%
R
1
= H, R
2
= n-Pr, R
3
= p-MeOC
6
H
4
: 66%
R
1
= H, R
2
= (CH
2
)
2
OTBS, R
3
= p-MeOC
6
H
4
: 48%
R
1
= Me, R
2
= TMS, R
3
= p-MeOC
6
H
4
: 54%
R
1
= MeO, R
2
= TMS, R
3
= p-MeOC
6
H
4
: 54%
R
1
= Cl, R
2
= TMS, R
3
= p-MeOC
6
H
4
: 52%
R
1
= MeO, R
2
= TMS, R
3
= Me: 55%
Scheme 3.33