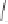Chemistry Reference
In-Depth Information
Co
2
(CO)
8
(10 mol%)
Toluene, 65 °C
Z
Z
O
R
1
R
1
H
MS4A pretreated at 200 °C
for 3 h under CO atmosphere
R
2
R
2
Trans
or
cis
isomer
Z = C(CO
2
Et)
2
,R
1
= H, R
2
= OMe: 90% (
trans
isomer)
Z = C(CO
2
Et)
2
,R
1
= OMe, R
2
= H: 88% (
cis
isomer)
Z = NBoc, R
1
= H, R
2
= H: 70% (
trans
isomer)
O
The same as above
H
OH
OH
70%
Scheme 3.15
Chung reported two protocols using non-carbonyl cobalt complexes as carbonyl complex
precursors. (Indenyl)Co(octa-1,5-diene) was used as relatively stable Co(I) complex.
16
Monosubstituted acetylenes even with a hydroxy group were good substrates and just
1 mol% cobalt catalyst realized an excellent yield. As for disubstituted acetylenes, the yield
was moderate when using 5 mol% cobalt catalyst (Scheme 3.16).
O
R
1
R
1
(Indenyl)Co(cod) (1-5 mol%)
+
DME, 100 °C
R
2
R
2
CO (15 atm)
R
1
= Ph, R
2
= H: 93%
R
1
= n-C
6
H
13
, R
2
= H: 95%
R
1
= CH
2
OH, R
2
= H: 96%
R
1
= (CH
2
)
4
OH, R
2
= H: 97%
R
1
= Ph, R
2
= Ph: 59%
R
1
= Ph, R
2
= Me: 53%
With norbornadiene
R
1
= Ph, R
2
= H: 82%
R
1
= n-C
6
H
13
, R
2
= H: 95%
With norbornene
Scheme 3.16
Another approach was the use of the more stable Co(II): Co(acac)
2
was reduced by
NaBH
4
for the preparation of an active catalyst.
17
Compared with Co
2
(CO)
8
, the cobalt
carbonyl complex precursors are easy to handle, but a pressurized condition of CO
is required. Various monosubstituted acetylenes were submitted, and ethyl propiolate
also reacted with norbornadiene, yet in a low yield (Scheme 3.17). In the reaction of