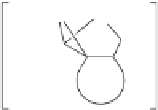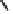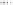Chemistry Reference
In-Depth Information
OC
OC
CO
TS1eq
cis
CO
Co
CO
2.210/2.211
26.3
OC
CO
TS1ax
cis
Co
MeO
2
C
H
CO
23.4
3.220
2.848
TS1eq
tr
MeO
2
C
CH
3
Cax
TS1ax
tr
24.3
D
cis
OC
OC
Co
CO
21.7
2.252/2.253
2.930
CO
Co
OC
CO
MeO
2
C
CO
H
Ceq
tr
OC
9.1
Cax
9.0
MeO
2
C
CH
3
OC
CO
Ceq
tr
Ceq
cis
D
tr
CO
2.267/2.276
2.929
Co
8.5
3.3
MeO
2
C
CO
H
D
cis
0
Ceq
cis
D
tr
OC
CO
OC
OC
Co
CO
OC
OC
CO
OC
OC
CO
CO
CO
CO
Co
Co
Co
2.005
1.986
MeO
2
C
CO
H
MeO
2
C
CO
H
MeO
2
C
H
MeO
2
C
H
1.977
1.932
TS1eq
cis
TS1eq
tr
TS1ax
tr
TS1ax
cis
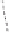
Figure 2.9
Energy diagram for the alkene insertion step in the PK reaction of methyl propy-
noate with ethylene.
Tab l e 2 . 2
Correlation of the diastereoselectivity of intramolecular PK reactions and the
MM-computed energies of the corresponding intermediate metallacycles (
D
).
Co
2
(CO)
8
Me
3
NO
(OC)
3
Co
Co(CO)
2
+
O
O
H
H
D
Exo
Endo
H
exo
(metallacycles)
H
endo
−
H
endo
−
H
exo
Reaction
exo:endo
(exp.)
(products)
TMS
(R
=
H) 72:28
−
0.3
0.1
TMS
(R
MOM)
100:0
=
−
1.5
1.8
O
RO
RO
(cis) 92:8
−
1.9
2.5
HO
HO
O
(trans) 75:25
−
2.1
1.7
(R
=
H) 39:61
−
1.1
0.6
TBSO
R
TBSO
R
(R
=
Me) 77:23
−
1.2
1.2
O
(R
=
TMS) 96:4
−
3.1
4.8
(R
=
H) 50:50
−
0.2
0.9
TBSO
TBSO
R
R
(R
TMS)
100:0
=
−
3.7
1.7
TBSO
TBSO
O