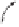Chemistry Reference
In-Depth Information
OC
OC
CO
TS1eq
cis
CO
Co
2.220/2.218
CO
Co
22.0
OC
CO
TS1ax
tr
MeO
2
C
CH
3
TS1eq
tr
20.6
CO
3.115
3.035
20.0
MeO
2
C
CH
3
Cax
cis
D
cis
TS1ax
cis
18.3
OC
OC
CO
CO
Co
2.213/2.198
CO
Co
OC
CO
MeO
2
C
CH
3
OC
2.904
3.255
Cax
tr
4.8
Cax
cis
MeO
2
C
CH
3
4.5
Cax
tr
D
tr
OC
OC
Co
CO
4.4
Ceq
tr
Ceq
cis
2.231/2.237
2.922
3.0
2.6
D
cis
MeO
2
C
CO
CH
3
0
D
tr
Ceq
tr
OC
CO
OC
OC
CO
OC
OC
CO
OC
OC
CO
OC
CO
CO
CO
CO
Co
Co
Co
Co
CO
2.292/2.282
2.938
Co
1.975
1.913
MeO
2
C
CH
3
MeO
2
C
CO
CH
3
MeO
2
C
CO
CH
3
MeO
2
C
CH
3
MeO
2
C
CO
CH
3
1.980
1.973
Ceq
cis
TS1eq
cis
TS1eq
tr
TS1ax
tr
TS1ax
cis
Figure 2.8
Energy diagram for the alkene insertion step in the PK reaction of methyl butynoate
with ethylene.
On this basis, the theoretical analysis was focused on calculating the transition states for
the olefin insertion starting from the three favored isomers (
Ceq
cis,
⊥
,
Ceq
trans,
⊥
and
Cax
,
Figure 2.6), observing interestingly that the system did indeed show a Curtin-Hammett
behavior, the axial coordination giving place to the lowest transition state for the insertion
(
TS1ax
tr
) despite the olefin complex (
Cax
) itself lying significantly higher in energy than
the equatorial analogues. Having in consideration the two possible TS's for insertion of the
olefin from the axial position (
TS1ax
cis
and
TS1ax
tr
), as well as the ones for insertion from
both equatorial positions (
TS1eq
cis
,
TS1eq
tr
), the experimentally observed regioselectivity
for these kind of complexes was correctly reproduced (Figure 2.7).
This approach has been extended to explain the regioselectivity of three differently
polarized alkynes as propyne, methyl propiolate and methyl butynoate
30
(Figures 2.8 and
2.9), thus integrating the former, polarization-based analysis (see above). The results show
that, although in all cases the insertion step seems to take place from the complex with
the olefin in pseudoaxial position (
Cax
), the different polarizations of the acetylene unit
in these complexes played a fundamental role in determining which end of it was to be
involved in the C-C bond formation event (
TS1ax
cis
or
TS1ax
tr
).
2.4.3
Stereoselectivity
Despite the important advances made in the last years in stereoselective Pauson-Khand
reactions, only a few attempts to computationally model this kind of reactions have been
made. Pioneering studies dealing with diastereoselective, stoichiometric versions (with
chiral substrates or chiral auxiliaries) were published prior to the complete DFT study of
the mechanism, modeling the stereo-determining step by simple MM or semi-empirical
methods.