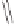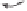Chemistry Reference
In-Depth Information
TS1eq
tr
15.2
TS1ax
13.8
CO
OC
OC
OC
CO
OC
OC
CO
TSeq
tr
,ax
5.7
CO
CO
Co
Co
Co
TS
tr⁄⁄,
4.7
H
CH
3
H
CO
CH
3
H
CO
CH
3
Ceq
tr⁄⁄
3.4
Cax
Ceq
cis
Ceq
tr
Cax
2.8
Ceq
tr
0.0
Figure 2.6
Computational analysis of the interconversion between the different olefin-
coordinated complexes
C
for the PKR between propyne and ethylene.
although with no success, this making apparent that a more complete theoretical analysis
was necessary for obtaining a good model for the regioselectivity with polarized alkynes.
28
In a more detailed study, focused on the olefin insertion step,
29
the possible modes
of olefin coordination in a Co
2
(CO)
5
(propyne)(ethylene) complex were analyzed. After
demonstrating that the perpendicular conformation of the olefin is preferred, the barriers
for olefin rotation and for the Co(CO)
2
(alkene) unit pseudorotation were determined. The
low barriers found demonstrated that the initial coordination position is not the key issue
for determining the regioselectivity of the reaction (Figure 2.6).
OC
OC
CO
TS1eq
cis
CO
CO
Co
OC
CO
2.185/2.177
18.8
TS1ax
cis
Co
18.6
1.513
H
CH
3
OC
2.816
Cax
H
3.237
CH
3
TS1eq
tr
D
cis
16.6
OC
OC
CO
TS1ax
tr
15.2
2.246/2.237
2.965
CO
Co
OC
CO
Co
H
CO
CH
3
1.516
CO
Ceq
cis
H
CH
3
D
tr
4.2
Cax
OC
CO
CO
2.265/2.251
2.935
2.0
Co
Ceq
cis
Ceq
tr
0.5
D
cis
1.4
H
CO
CH
3
0
D
tr
Ceq
tr
OC
OC
CO
OC
OC
CO
OC
CO
OC
OC
Co
CO
CO
CO
CO
Co
Co
2.010
Co
1.995
1.934
1.937
H
CH
3
H
CH
3
H
CO
CH
3
H
CO
CH
3
1.992
1.979
1.953
1.960
TS1eq
tr
TS1eq
cis
TS1ax
tr
TS1ax
cis
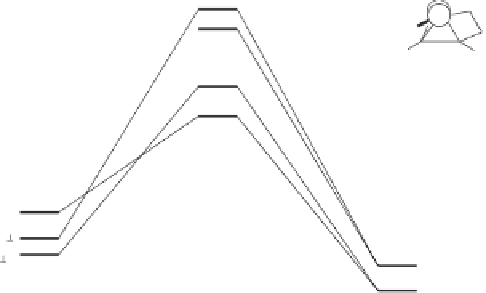
Figure 2.7
Analysis of the transition states for the olefin insertion step (
TS1
) as a function of
the different isomers of intermediate
C
, in the PKR of propyne and ethylene.