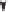Chemistry Reference
In-Depth Information
Co
2
(CO)
8
+
R
O
2 CO
R
Co
Co
CO
R
I
R
O
R
Co
Co
Co
Co
V
R
II
O
Co
Co
Co
Co
III
R
R
IV
Scheme 2.16
Catalytic cycle for the PKR as proposed by Verdaguer, Riera
et al.
24
aspects, as regio- and stereoselectivity. This pioneering work has thus turned into a reference
for any computational approach to the Pauson-Khand reaction.
In Scheme 2.17 and Figure 2.3, the reaction pathway as determined by Nakamura and
coworkers is shown. Following their results, the mechanism can be clearly divided in two
parts, the first (from the starting materials to intermediate
C
, before the alkene insertion)
being endothermic and the second (from the alkene insertion to the ending products) being
strongly exothermic and presumably irreversible.
Although the authors do not delve deeply into the exact mechanism of the carbonyl
substitution by olefin, they assume a dissociative process has taken place, through the
intermediate unsaturated species
B
. As shown by calculations, formation of
B
would
be the most energetically demanding step of the whole process, which would explain the
difficulties encountered up to that moment to experimentally isolate or even detect any of the
reaction intermediates. Even if the reaction was not to proceed through
B
but through a lower
energy associative transition state, the significant (ca. 15 kcal
mol
−
1
) difference between
A
and
C
would make the latter at best hard to isolate or even observe by experimental means.
·