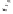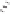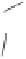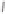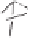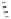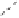Chemistry Reference
In-Depth Information
formation step should become irreversible. This prediction, fully confirmed by theoretical
studies, is in agreement with the fact that no equilibration of PK adducts has ever been
observed.
R
R
R
CO
CO or
Lewis base
CO
L
OC
CO
OC
OC
CO
Co
Co
CO
OC
Co
Co
CO
OC
Co
OC
Co
OC
OC
OC
Thermoneutral
Very exothermic
H
H
H
Scheme 2.12
Irreversibility of the cobaltacycle formation process.
2.2.3 CO Insertion
The coordinatively saturated cobaltacycles resulting from the previous step in the PKR
mechanism have never been detected from an experimental point of view. According to
the Magnus mechanism and to subsequent theoretical studies, one of the CO ligands in the
coordination sphere of the active Co atom is next inserted into one of the carbon-cobalt
bonds, installing in this manner the future carbonyl group of the cyclopentenone product.
According to precedents with geometrically unconstrained systems, this insertion step
should involve very low activation energy, since the transition state geometry is achieved
by simple modification of a rather flexible C-Co-CO angle.
Although CO insertion could take place in any of the two carbon-cobalt sigma bonds in
the cobaltacycle (Scheme 2.13), it has always been considered that insertion takes place in
the C(sp
3
)-Co bond. Yamanaka and Nakamura
16a
studied this problem from the theoretical
point of view (see below) and found that while this process is in fact more favorable, the
alternative insertion in the C(sp)-Co bond involves a transition state only 4 kcal
mol
−
1
higher in energy. In any case, the nature of the bond experiencing CO insertion should have
no effect on the nature of the reaction product since both acylcobaltacycles should lead
upon reductive elimination to the same cyclopentenone.
·
CO
R
R
L
CO
OC
L
OC
Co
Co
O
CO
OC
Co
OC
Co
OC
OC
H
H
R
Favored
CO
OC
L
Co
CO
OC
Co
OC
H
O
R
R
CO
OC
L
OC
L
L = CO or Lewis base
Co
CO
Co
CO
OC
Co
OC
Co
OC
OC
H
H
Disfavored
Scheme 2.13
Possible routes for the CO insertion.