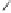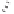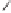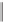Chemistry Reference
In-Depth Information
H
H
O
CO
OC
OC
CO
CO
Co
OC
Co Co
CO
OC
Co
OC
OC
R
H
H
R
R
Regioisomers
H
H
O
CO
OC
OC
CO
CO
Co
OC
Co Co
CO
OC
Co
R
OC
OC
R
R
H
H
R
H
O
CO
OC
CO
OC
R
CO
Co
OC
Co Co
CO
OC
Co
OC
OC
H
H
H
R
H
Enantiomers
R
H
O
CO
OC
CO
OC
R
CO
Co
OC
Co
Co
CO
OC
Co
OC
OC
R
H
H
H
H
Scheme 2.11
Importance of alkene rotation processes.
the course of cobaltacycle formation (and of the whole PKR), the situation may well
not be this simple. Since the energetic cost of olefin rotation or Co(CO)
2
(alkene)
pseudorotation should be small in comparison with cobaltacycle formation, this last
process must be governed (assuming kinetic control) by the Curtin-Hammett princi-
ple, so that only the relative energies of the different transition states connecting the
conformers of the Co
2
(CO)
5
(alkyne)(alkene) complex with the product cobaltacycle(s)
determine the course of the reaction. Thus, irrespective of the nature (pseudoaxial or
pseudoequatorial) of the vacant coordination site generated by CO loss, of the initially
formed olefin complex, and of the nature (pseudoaxial or pseudoequatorial) of the
most stable olefin complex, cobaltacycle formation is governed by the kinetics of the
metathesis step.
Simple thermochemical considerations based on bond energies, confirmed by the avail-
able theoretical studies (see below) indicate that formation of a coordinatively unsaturated
cobaltacycle, like those depicted in Schemes 2.10 and 2.11, should be essentially thermoneu-
tral and this can raise a question over the possible reversibility of this step. It is important to
consider, however, that the coordinatively unsaturated cobaltacycle intermediate will react
without any energy barrier with CO or with Lewis bases present in the reaction medium to
afford a much more stable species (Scheme 2.12). In this manner, the overall cobaltacycle