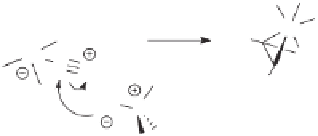Chemistry Reference
In-Depth Information
control, efforts have been devoted to developing chemical methodologies that could render
this initial dissociative step irreversible.
The most successful approach in this direction has been based on the use of amine
oxides as chemical promoters of CO dissociation. Following early work on oxygen atom
transfer to metal carbonyls,
7
Schreiber
8
and Jeong
9
independently reported very significant
accelerations of both intramolecular and intermolecular PKR through the use of amine
oxides as
N
-methylmorpholine oxide (NMO) and trimethylamine
N
-oxide (TMAO) as
promoters for the reaction (Scheme 2.5).
1) Co
2
(CO)
8
2)
Thermal
31%
, isooctane
or
O
O
O
1) Co
2
(CO)
8
, CH
2
Cl
2
2) 6eq NMO, rt
Promoted
85%
OTHP
OTHP
H
H
EtOOC
EtOOC
TMAO (3eq)
O
O
2
, CH
2
Cl
2
, r.t.
92%
EtOOC
EtOOC
Co
2
(CO)
6
Scheme 2.5
Use of
N
-oxides as promoters for the PKR.
In these cases, the mechanism of activation by the
N
-oxide would be that proposed
by Alper for the
N
-oxide activation of metal carbonyls:
7b
a nucleophilic attack of the
N
-oxide on one of the carbonyl carbons to generate the goods, leaving group CO
2
and
a coordinatively unsaturated metal carbonyl (Scheme 2.6). Probably depending on the
nature of the reacting alkene, the
N
-oxide promoter and the employed reaction conditions,
this coordinatively unsaturated species reacts preferentially with the excess
N
-oxide, the
tertiary amine generated in its deoxygenation, or directly enters the reaction pathway
through coordination of the alkene.
Co
Co
Co
Co
Co
Co
R
R
R
CO
2
O
C
C
O
R
R
R
N
O
O
N
N
Scheme 2.6
Mechanism for the activation of PK substrates by
N
-oxides (formal charges made
explicit for clarity).
From a practical perspective, the promotion of the PKR by
N
-oxides has become a well
established and much used methodology. The possibility of selectively inducing decar-
bonylation at one of the enantiotopic cobalt atoms of a dicobalt hexacarbonyl process in
view of controlling the stereochemical course of the reaction with the use of enantiopure
brucine
N
-oxide (Figure 2.1) has been explored by Kerr and co-workers.
10