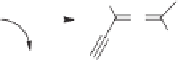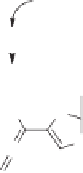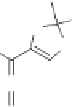Chemistry Reference
In-Depth Information
The Au(I)-catalyzed hydrative rearrangement of 1,1-diethylcarbinol acetates is an in-
triguing methodology employed in the synthesis of cyclopentenones.
82
This reaction is
strongly dependent on the reaction conditions as shown in Scheme 10.47.
O
OAc
C
OAc
AuCl(PPh
3
)/AgSbF
5
CH
2
Cl
2
/Water, 0 °C
R
AuCl(PPh
3
)/AgSbF
5
CH
2
Cl
2
/Water, r.t.
R
OAc
R
O
163
162
161
R= Bn
163a
82% yield
R= cyclohexyl
163b
60% yield
Scheme 10.47
Au(I)-catalyzed hydrative rearrangement of 1,1-diethylcarbynol acetates.
The Au(I)-catalyzed hydrative rearrangement reaction begins with the formation of
164
via an Au catalyzed [3,3]-sigmatropic shift of the acetate group (Scheme 10.48). Due to the
extraordinary alkynophilicity of gold, Au coordinates with the triple bond of
164
, which in-
duces oxacyclization/1,3-dioxolium ion generation via an internal nucleophilic attack by the
carbonyl oxygen of the acetoxy group at the C-2 position of the allene to furnish
165
.Next,a
molecule of water is expected to be involved at this stage to form an intermediate
166
, which
is converted into
167
by simple deprotodemetalation. Interestingly, Au(I) further activates
the allene functionality, which induces the sequential 1,3-dioxole ring opening and the 5-
endo-dig carbocyclization to furnish the gold-coordinated cyclopentenone
168
. The latter,
upon protodemetalation, renders the cyclopentenone
163
as illustrated in Scheme 10.48.
O
+
OAc
R
O
Au(I)
C
R
OAc
R
Au(0)
Au(I)
165
Au(I) H
2
O
162
164
OH
O
O
R
O
R
O
OH
2
+
R
OAc
O
C
Au(I)
C
(0)Au
(0)Au
168
166
167
Au(I)
O
R
OAc
163
Scheme 10.48
Mechanism for
the Au(I)-catalyzed hydrative rearrangement of 1,1-
diethylcarbynol acetates.