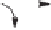Chemistry Reference
In-Depth Information
N
2
N
2
O
OEt
O
OEt
O
Rh
2
(acam)
4
CH
2
Cl
2
,r.t.
O
O
H
CO
2
Et
120
76% yield
119
118
Scheme 10.34
Rh(II)-catalyzed synthesis of cyclopentenones from
α
,
β
-unsaturated carbonyl
compounds.
10.6 Other Methodologies
The selective monodecarbonylative coupling of cyclobutenediones with alkenes is yet an-
other method employed in the synthesis of cyclopentenones. Kondo and co-workers discov-
ered the ruthenium-catalyzed reconstructive synthesis of cyclopentenones by an unusual
coupling reaction of cyclobutenediones with alkenes, involving a C-C bond cleavage.
74
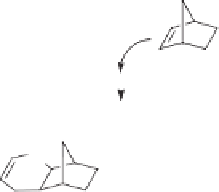
The suggested mechanism (Scheme 10.35) for the reaction is as follows: first, the oxidative
addition of cyclobutenedione
121
into an active ruthenium complex occurs selectively at
the C2-C3 bond under the direction of an alcoxy substituent to generate a ruthenacyclopen-
tenedione intermediate. It is important to use the appropriate carbon monoxide pressure
(3 atm) in order to control the selective mono-decarbonylation of the ruthenacyclopentene-
dione leading to a ruthenecyclobutenone intermediate while concomitantly suppressing
the complete decarbonylation leading to an alcoxyalkyne and CO. Next, stereoselective
cis-carboruthenation of 2-norbenene
124
and reductive elimination with retention of stere-
ochemistry renders the corresponding exo-cyclopentenone
127
.
R
1
O
R
1
O
R
1
O
O
[Ru]
[Ru]
[Ru]
123
O
R
2
R
2
R
2
O
O
CO
O
121
122
124
[Ru]
OR
1
OR
1
R
1
O
R
2
R
2
[Ru]
or
R
2
O
O
[Ru]
O
126
127
125
Scheme 10.35
Proposed mechanism for the Ru-catalyzed monodecarbobylation of cyclobu-
tadienones.
This methodology affords the final cyclopentenones in moderate to good yields and with
total control of stereoselectivity as shown in Scheme 10.36.