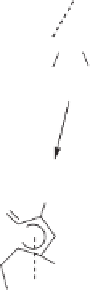Chemistry Reference
In-Depth Information
When the reaction proceeds intramolecularly via an alkyne substituent bonded to the
carbine moiety by means of the alkoxy substituent, the reaction furnishes a cyclopentenone-
fused oxygen heterocycle as shown in Scheme 10.30. This represents a nice extension of
this methodology.
65
Cr(CO)
5
O
O
Ph
H
Δ
74% yield
93:7 d.r.
O
Ph
103
102
Scheme 10.30
Intramolecular cylization of cyclopropyl chromium complexes.
In the proposed mechanism (Scheme 10.31), the alkyne inserts into the chromium com-
plex with formation of a metallacyclobutene
105
, which is converted into the vinylcarbene
complex
106
. This mechanism is similar to mechanism described for the Dotz reaction.
66
Final conversion into a cyclopentadienone then occurs via a 1,5-alkyl shift, followed by
CO insertion, conversion to the pentadienyl complex, ring closure and fragmentation with
ethylene loss. Subsequent reduction with Cr(0) in water renders the cyclopentenone.
R
R
R
R
R
Cr(CO)
5
74
(OC)
4
Cr
Cr(CO)
4
-CO
OMe
R
OMe
OMe
99
104
105
(CO)
3
Cr
Cr(CO)
4
R
O
Cr(CO)
4
R
MeO
R
R
106
MeO
R
MeO
R
107
108
R
R
O
O
O
O
R
R
+
R
OMe
R
OMe
H
2
O
+Cr(0)
R
R
Cr(CO)
3
OMe
OMe
Cr(CO)
3
101
111
109
110
Scheme 10.31
Proposed mechanism for cyclopentenone synthesis by cyclization of cyclo-
propyl chromium complexes.