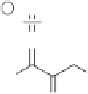Chemistry Reference
In-Depth Information
10.3
[3
+
2] Strategies for the Synthesis of Cyclopentenones
10.3.1
Iron Promoted Reactions
The use of [3
2] cycloadditions is another commonly employed strategy for the synthe-
sis of cyclopentenones using organometallic compounds. Specifically, the iron carbonyl-
promoted cyclocouplings between secondary dibromoketones and enamines provides a
synthetic route to
+
-dialkylated cyclopentenones (Scheme 10.15). The ketone is con-
verted to the iron enolate
49
by diiron nonacarbonyl, and undergoes cycloaddition with
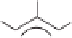
nucleophilic alkenes such as
50
, affording the cyclopentenones. The use of morpholino-
enamines leads to products that rapidly eliminate morpholine, providing cyclopentenones
in a single one-pot procedure.
24-27
α
,
α
O
N
50
O
FeL
n
O
O
Fe
2
(CO)
9
(
48
)
benzene
Me
Me
Me
Me
Br
Br
100% yield
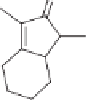
49
47
51
Scheme 10.15
Iron catalyzed synthesis of cyclopentenones.
Another important consideration for this reaction is that enamines of both aldehydes
and ketones can be used, and bicyclic cyclopentenones can be easily obtained using cyclic
enamines as a starting material. However, one important limitation of this methodology is,
in fact, the impossibility of preparing
α
β
-unsubstituted cyclopentenones.
A fascinating variation of this method employs iron complexes such as
52
, with ketenes
such as diphenyl ketene
53
(Scheme 10.16).
28, 29
The generated iron complex can then be
easily cleaved using a variety of oxidative and non-oxidative procedures.
,
Me
(OC)
2
CpFe
52
(OC)
2
CpFe
(OC)
2
CpFe
Ph
C
Ph
+
Ph
55
Me
Ph
Me
Ph
Ph
O
O
54
53
C
O
74% yield
Scheme 10.16
Synthesis of cyclopentenones from ketenes.