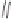Chemistry Reference
In-Depth Information
O
O
OAc
O
Cl
Br
(CH
2
)
5
CO
2
Me
PPh
3
O
O
OAc
O
O
HO
OHC
2
O
O
C
5
H
11
8
7
Punaglandin 4
Scheme 10.3
Synthesis of cyclopentenones via an intramolecular Wittig reaction.
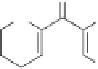
With the advent of organocatalysis, the Nazarov reaction
11
has emerged as a com-
mon method for the synthesis of cyclopentenones. The Nazarov reaction is an acid-
induced cyclization of allyl vinyl and divinyl ketones that yields substituted cyclopen-
tenones. An elegant example of this reaction was described by Rueping and co-workers
(Scheme 10.4).
122
O
O
R
1
O
R
1
10%
11
O
86-98% ee
R
2
R
2
10
9
O
O
11
=
P
NH
O
SO
2
CF
3
Scheme 10.4
Nazarov reaction reported by Rueping.
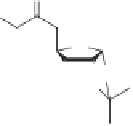
The final example in this category of reactions is the preparation of cyclopentenones by
intramolecular 1,5-C-H insertions.
13
Alkylidene carbenes undergo regio- and stereoselec-
tive intramolecular C-H insertion reactions affording cyclopentenes. Alkylidene carbenes
can be generated from alkynes under flash vacuum pyrolysis conditions by a reversible
1,2-shift. This strategy has been used to prepare a key intermediate in the synthesis of
(
)-isocomene, as illustrated in Scheme 10.5.
14
±
OAc
OAc
Me
Me
Me
OAc
Me
Me
Δ
Me
Me
Me
Me
Me
O
Me
Me
H
15
H
O
O
14
13
(+)-isocomene
12
Scheme 10.5
Synthesis of cyclopentenones via 1,5-C-H insertion.