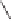Chemistry Reference
In-Depth Information
A practical and realistic asymmetric PKR catalyzed by heterogeneous catalysts was re-
ported by Chung's group.
13
They studied a
Co
2
Rh
2
-catalyzed asymmetric PKR in the
presence of a chiral diphsosphine, which included (
R
)-BINAP, (
R
)-tol-BINAP, (
R
,
R
)-
NORPHOS, (
R
)-DIOP and (
S
,
S
)-BDPP (Scheme 9.14). Based on the ee and % yield,
(
S
,
S
)-BDPP was choice. Using crotonaldehyde as a CO source, a highly eantioselective
reaction was observed under the optimized reaction conditions (enyne (0.96 mmol), croton-
aldehyde (2.4 mmol), (
S
,
S
)-BDPP (0.11 mmol),
Co
2
Rh
2
on charcoal (0.05 g, total weight
of metals 12.2 mg), THF (5 mL), 18 h, 130
◦
C).
Ph
Ph
Co
2
Rh
2
(4
mol%), L* (12 mol%)
O
O
O
2.5 equiv crotonaldehyde
∗
ee (%)
Yield (%)
"L*"
(
R
)-BINAP
(
R
)-tol-BINAP
(
R,R
)-NORPHOS
(
R
)-DIOP
(
S,S
)-BDPP
93
91
83
90
91
79
73
-
34
84
Scheme 9.14
Various enyne substrates for the intramolecular PKR were tested under the optimized
reaction conditions (Table 9.9). The yields were high (75-94%) and the ee values were mod-
erate to high (51-87%). In some cases, the observed ee values were comparable to those
obtained in homogeneous catalysis using rhodium catalysts derived from [RhCl(CO)
2
]
2
/(
S
)-
BINAP and AgOTf.
41
According to their study on the reusability of the catalytic system, the
yields and ee values were retained over at least five runs. However, the chiral diphosphine
Tab l e 9 . 9
R
R
Z
Z
O
Substrate
Product
Z
O
O
NTs
C(CO
2
Et)
2
C(CO
2
Et)
2
R
Yield (%)
ee (%)
Ph
Bu
Ph
Me
Ph
91
75
94
93
94
84
62
85
87
51