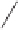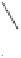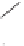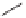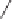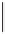Chemistry Reference
In-Depth Information
Tab l e 9 . 5
Substrate
Product
Yield (%)
EtO
2
C
EtO
2
C
89
O
EtO
2
C
EtO
2
C
Me
EtO
2
C
Me
EtO
2
C
90
O
EtO
2
C
EtO
2
C
O
+
Ph
88
Ph
O
OH
+
82
OH
9.4 Bimetallic Nanoparticle Catalysts
A representative structure of bimetallic nanoparticle catalyst systems displays a core/shell,
cluster-in-cluster or alloy (Figure 9.2). Besides the original function that can act as a
catalyst in two consecutive reactions, these systems may provide a way to enhance the
activity relative to the monometallic catalysts.
Immobilized Heterobimetallic Ru/Co Nanoparticle
25
9.4.1
Heterobimetallic ruthenium/cobalt nanoparticles immobilized on charcoal (
RuCNC
)were
developed as catalysts in the PKR using pyridylmethyl formate as a substitute of carbon
monoxide (Scheme 9.11).
25
Among organic substitutes screened in the PKR,
33
pyridyl-
methyl formate was of choice. When either ruthenium carbonyl or colloidal cobalt nanopar-
ticles were used as a catalyst in the presence of pyridylmethyl formate, no reaction product
was observed.
B
A
A
B
A
A/B
A
A
Random alloy
Cluster-in-cluster
Core/shell
Figure 9.2
[36a] Reproduced by permission of EDP Sciences.