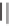Chemistry Reference
In-Depth Information
N
N
N
N
N
N
N
N
N
N
mpg-C
3
N
4
N
N
N
N
N
N
N
N
N
N
N
NN
N
NN
N
Figure 9.1
[25] Reproduced by permission of the Royal Society of Chemistry.
CO
2
. They discovered that phenol, biphenyl and CO could be generated by the reaction
of benzene and CO
2
in the presence of
mpg-C
3
N
4
. The generated CO was quenched in
a PK-type reaction (Scheme 9.5). When benzene, 1-hexene and dimethylacetylene-1,2-
dicarboxylate (DMAD) were reacted in the presence of
mpg-C
3
N
4
at 150
◦
C under 10
bar of CO
2
for 2 d, 70% of the PK product and 30% of a Diels-Alder reaction product
between DMAD and biphenyl were observed. Although the experimental details have not
been reported, it is reasonable to say that the reaction is promoted.
O
CO
2
Me
MeO
2
C
C
2
Me
CO
2
(10 bar),
mpg-C
3
N
4
48 h, 150
MeO
2
C
+
+
+
°
C
CO
2
Me
MeO
2
C
C
4
H
9
Scheme 9.5
9.3 Transition Metal Nanoparticle Catalyst
When bulk metallic cobalt was used as a catalyst in the PKR, the catalytic system was
effective but it still required harsh reaction conditions, presumably due to the low surface-to-
volume ratio of cobalt. However, if cobalt nanoparticles were used as catalysts, the surface-
to-volume ratio of cobalt and the number of active sites dramatically increased because
smaller particles have more edges and kinks than larger particles.
23
Small nanoparticles
provide a tremendous driving force for diffusion especially at elevated temperatures. In
addition, sometimes an inexplicable synergistic effect is also expected.
24
For these reasons
researchers have dedicated themselves to using transition metal nanoparticles as catalysts.
Since the first cobalt nanoparticle was used as a catalyst in the PKR, heterobimetallic
nanoparticle systems such as Co/Ru,
25
Co/Rh
13, 26
and Co/Pd
27
have been developed. We
will discuss each topic briefly.