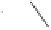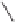Chemistry Reference
In-Depth Information
EtO
2
C
EtO
2
C
EtO
2
C
Cat
+
O
EtO
2
C
EtO
2
C
EtO
2
C
68%
OH
OH
O
Ph
2
P
O
OH
Co(CO)
3
Co(CO)
3
Ph
2
O
O
Cat
OH
O
OH
OH
O
O
OH
OH
Scheme 9.2
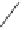
In the catalytic reaction, along with the regular PKR product, an Alder-ene-type byprod-
uct was obtained under all reaction conditions. They presented only one example (the best
yield of the PKR product: 68%). Those catalytic cycles work under mild reaction conditions
(5 mol% Co, 1.05 bar CO, THF, 70
◦
C, 24 h; 5 mol% of Co dimer, 1 atm CO, THF, 70
◦
C,
24 h) compared to those of the metallic cobalt system.
9.2.2 Bulk Cobalt as a Catalyst
11,17
The first metallic cobalt catalyst for the PKR was prepared by decomposing Co
2
(CO)
8
in
a refluxing toluene solution or using the conventional impregnation method that adopts
cobalt nitrate as a cobalt source with mesoporous silica such as SBA-15 and MCM-41.
11
The choice of mesoporous silica as a support was due to its large surface areas and porous
structures.
16
The metallic cobalt immobilized on mesoporous silica was quite effective for
the intramolecular PKR although harsh reaction conditions were necessary (Scheme 9.3).
EtO
2
C
EtO
2
C
Co/silica (9~10 wt%)
O
20 atm CO, 130
°
C
EtO
2
C
EtO
2
C
0.5 h, THF
95%
Scheme 9.3
To obtain good results, the cobalt loading should be adjusted to 9-10 wt% and the reaction
temperature and the pressure of CO should be at least 130
◦
C and 20 atm, respectively.
The catalyst was air-stable and reusable, and exhibited an excellent catalytic performance
for many intramolecular PKRs (Table 9.1). The reaction gave good yields with mono-
substituted olefins regardless of the substitution pattern of alkynes, while the internal alkyne
substrate needed a relatively long reaction time. However, the catalytic system displayed