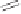Chemistry Reference
In-Depth Information
OEt
O
O
H
1
OH
H
2
H
1
H
2
OEt
O
O
H
2
O/THF, pTSA (cat.)
*
O
O
OH
O
O
70 °C, 20 h, 72%
126a,
H
1
=H
2
=β
127a
, H
1
=H
2
=β
127b,
H
1
=b, H
2
=α
125
126b,
H
1
=a H
2
=β
126c,
H
1
=H
2
=α
OH
OMe
OH
H
H
H
H
H
H
NaIO
4
O
O
+
H
2
O-MeOH
O
O
O
OH
O
O
O
O
O
127a
129
128
*Conditions for the tandem Pauson-Khand cyclization
1. Co
2
(CO)
8
(25 mol%), DME, CO (1 atm), hv (Q-Beam), 60 °C, 48 h, 55%,
126a:126b:126c
: = 2:3:1, d.r (
126b
) = 1:1.2
2. Co
2
(CO)
8
( 25 mol%), DME, CO (1 atm), 60 °C, 36h, 65%
126a:126b:126c
: = 1.5:1:trace, d.r (
126b
) = 2:1
3. Mo(CO)
6
(240 mol%), DMSO (10 eq), toluene, Ar, 90-100 °C, 4-8 h, 72%,
126a:126b:126c
= 1:2.5:trace, d.r (
126b
) = 1.5:1
4. [Rh(CO)
2
Cl]
2
(10 mol%), toluene, CO (1 atm), 100 °C, 28 h, 36%,
126a:126b:126c
= 1:1.2:trace, d.r (
126b
) = 5:3
5. [Ir(COD)Cl]
2
(20 mol%), Ph
3
P (40 mol%), CO (1 atm), toluene, reflux, 24 h, 76%,
126a:126b
= 4:1 d.r (
126b
) = 5:1
6. [RhCl(CO)dppp)
2
(20 mol%), CO (1 atm), CH
3
CN, reflux, 24h, 68%, 126a:126b = 7:1, d.r (126b) = 10:1
Scheme 8.19
The Rh and Ir mediated Pauson-Khand reaction for simple entry into [5.8.5]
ring systems.
this chapter illustrate the fundamental role the process has played in the total synthesis of
complex molecules of natural and computational interest.
69
Initially the process began by
employing cobalt as the metal mediator, after which the scope of the reaction was expanded
to include a variety of stoichiometric and catalytic conditions with different metal mediators
to improve diastereoselectivity and yield. Due to the robustness of the process to tolerate a
variety of functional groups, the use of the Pauson-Khand reaction will continue to make
significant contributions in synthetic organic chemistry for years to come.
Acknowledgements
The authors would like to dedicate this topic chapter in memory of Andrew J. Strom,
pre-pharmacy student at Concordia University Wisconsin.
References
[1] (a) Nicolaou, K.C., Vourloumis, D., Winssinger, N., and Baran, P.S. (2000) The art
and science of total synthesis at the dawn of the twenty-first century.
Angew. Chem.
Int. Ed.
,
29
, 44-122; (b) Bertz, S.H. (1981) The first general index of molecular
complexity.
J. Am. Chem. Soc.
,
103
, 3599-3601.