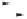
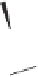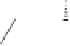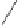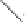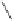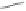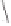Chemistry Reference
In-Depth Information
O
O
Co
2
(CO)
6
H
H
H
H
H
+
O
O
O
H
H
H
H
H
H
Conditions
Thermal: CH
3
CN, reflux, 15 min: 85% (5:1)
N-oxide promotion: NMO, CH
2
Cl
2
, r.t., 12 h: 70% (11:1)
Scheme 1.16
As discussed previously, conditions for use of gaseous olefins in the PK annulation
require more forcing conditions and generally lead to only low to moderate yields of
cyclopentenone products.
7
In relation to this, work in our own laboratory has formulated
optimised conditions with
N
-oxide promotion for the use of ethylene as the alkene reaction
partner under appreciably more moderate temperatures and pressures.
34a-c
Indeed, whilst
mild autoclave pressure (25-30 atm) and temperatures (40
◦
C) provided optimum yields in
most instances, it was also found that atmospheric pressures of ethylene at room temperature
also delivered the targeted cyclopentenones. It is also worth noting that both techniques
result in considerably cleaner product reaction mixtures than those obtained under the more
traditional thermal protocols. As shown in Scheme 1.17, these developments allowed us
to efficiently complete a total synthesis of the sesquiterpene, (
)-taylorione
13
;
34a,c
the
requisite chiral alkyne complex
14
was prepared in seven steps from the inexpensive and
readily available starting material, (
+
)-2-carene
15
, and, following successful application
of the
N
-oxide promoted PK conditions with ethylene, (
+
+
)-
13
was obtained in short order
and with high optical purity.
Co
2
(CO)
6
O
O
O
O
O
O
CH
2
15
14
13
(+)-taylorione
Conditions
Thermal: C
2
H
4
(50 atm), C
6
H
6
, 80 °C, 5 h: 38%
N-oxide promotion: C
2
H
4
(25 atm), Me
3
NO.2H
2
O, tol./MeOH, 40 °C, 24 h: 81%
N-oxide promotion at atmospheric pressure and r.t:
C
2
H
4
(bubbling), Me
3
NO.2H
2
O, C
6
H
6
/MeOH, r.t., 18 h: 41%
Scheme 1.17
In continuing efforts to further develop the PK reaction towards delivering cyclopen-
tenones from ethylene incorporation, when vinyl esters, such as vinyl benzoate
16
,
were employed with
N
-oxide promotion, the products which resulted were those where
the expected ester unit had been cleaved, presumably via an
in situ
reduction process
with low valent cobalt.
34d-f
This outcome clearly establishes an extremely mild and
utilisable protocol for employment of the vinyl ester as an ethylene equivalent, without the
requirement for handling of gaseous reagents. Moreover and as illustrated in Scheme 1.18,
the new vinyl benzoate technique is at least comparable with the optimum ethylene