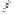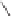Chemistry Reference
In-Depth Information
Tab l e 8 . 2
The Pauson-Khand cyclization in the total synthesis of (-)-incarvilline.
OMOM
OMOM
Co
2
(CO)
8
(1.05 eq)
Promoter, solvent
NTs
Me
OH
105
106
OMOM
Me
OMOM
H
H
H
H
O
+
HO
O
NTs
NMe
NTs
H
H
H
Me
Me
Me
107
109
(-)-Incarvilline
108
Yield (%)
Entry
Promoter (eq)
Solvent
Atm
T (
◦
C)
Time (h)
107
108
1
none
toluene
Ar
110
2
54
7
2
NMO (10)
CH
2
C1
2
Ar
rt
9
45
5
3
n
-BuSMe (3.5)
DCE
Ar
83
24
58
7
4
n
-BuSMe (3.5)
DCE
CO
83
2.5
66
7
5
t
-BuSMe (3.5)
DCE
Ar
83
2.5
62
6
6
t
-BuSMe (3.5)
DCE
CO
83
2.5
73
8
In this vein, a four -tep process shown in Scheme 8.16 from cyclopropane ester
110
furnished the required vinylcyclopropane substrate
114
in good overall yield. Model stud-
ies on simpler vinylcyclo substrates with various rhodium catalysts were performed to
establish the best conditions for construction of the bicyclic core of agarofuran. Reaction
I
MeO
2
C
MgBr
CO
2
Et
DIBAL-H
I
2
,PPh
3
imidazole
(73%, 2 steps)
CO
2
Et
Me
CO
2
Me
CuCN
BF
3
OEt
2
(66%)
NaH
(69%)
110
111
112
MeO
2
C
MeO
2
C
Me
Me
MeO
2
C
CO, [Rh(CO)
2
Cl]
2
LiCl, DMF
88%
[(3+2)+1] cycloaddition
(86%, dr 15:1)
113
114
Me
Me
Me
Me
O
MeO
2
C
O
Me
± -115
116
(±)-
α
-Agarofuran
Scheme 8.16
The Rh(I)-catalyzed Pauson-Khand reaction towards (
±
)-
α
-agarofuran.