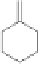Chemistry Reference
In-Depth Information
sequence from starting alkyne
87
in high yield. The reaction of the diastereomeric mixture
of yne-ols
89
with dicobalt octacarbonyl in ether afforded the alkyne complex followed
by the addition of TMANO in the presence of 4 A molecular sieves in toluene at
10
◦
C.
This process furnished the tricyclic enone
90
in 51% yield. The protection of the alcohol
with MOMCl and reduction of the ketone resulted in alcohol
91
in 90% yield. Alcohol
91
was then reacted under Mitsunobu conditions and this was followed by regeneration of the
secondary alcohol and oxidation using Dess-Martin conditions to provide the ketone
92
in
65% yield. The final steps to complete the total synthesis involved a two-step sequence to
cleave the protecting groups to provide (
−
±
)-8
α
-hydroxystreptazolone
93
in 82% yield.
8.10 The Formal Total Synthesis of (
±
)-
α
-and
β
-Cedrene
α
β
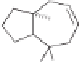
The tricyclic sesquiterpenes
-cedrene
104
are natural products from
the cedrene
102
family and possess the unique [5.3.1.0] tricylic skeleton. As a pioneer in
the development of the Pauson-Khand reaction,
2
Pauson and co-workers set up to employ
the cyclization reaction in efforts toward the formal total synthesis of cedrene analogs
103
and
104
53
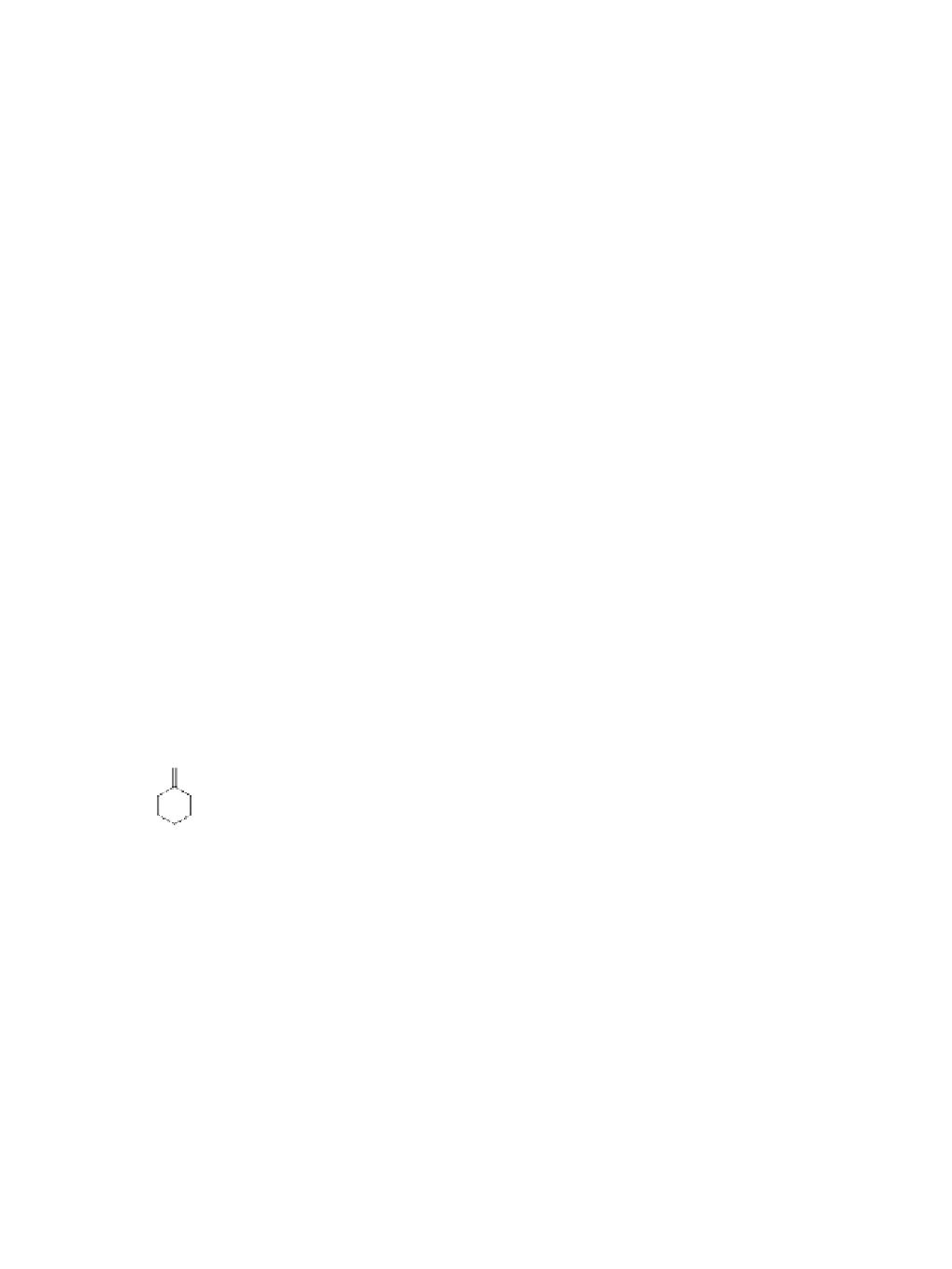
. As shown in Scheme 8.15, commercially available ketone
94
was taken
-cedrene
103
and
Me
O
O
O
LiAlH
4
,Et
2
O, 0 °C
, 1h
99%
Ph
3
P
+
CH
2
CH
3
Br
-
n
-BuLi, THF, 0
°C
, 2h
99%
O
3 steps (72%)
OEt
OEt
O
O
O
O
Me Me
O
O
Me Me
94
95
96
Me
Me
Me
AcC(N
2
)P(O)(OMe)
2
,K
2
CO
3
MeOH, rt, 5d
81%
Dess-Martin
10% CH
3
CN in DCM
97%
O
Co
2
(CO)
8
, petrol, rt, 2h
OH
H
99%
O
O
Me Me
O
O
Me Me
O
O
Me Me
98
99
97
Me
Me
Me
O
O
Pauson-Khand conditions(1-4)
O
+
O
O
O
Co
2
(CO)
6
O
O
Me
Me
Me
Me
Me Me
Conditions
1. 9 eq. TMANO 2H
2
O, acetone, rt 16h, 91%
2. 8 eq. NMO H
2
O, DCM, rt, 16h, 84%
3. 4.3 eq. nBuSMe, 1,2-DCE, 83
°
C, 30 min, 95%
4.3.6 eq. polymer-supported sulfide, 1,2-DCE, 83 °C, 30 min, 80%
101a
101b
100
Me
Me
Me
O
O
+
O
H
H
Me
Me
Me
Me
Me
Me
104
103
102
α
-Cedrene
β
-Cedrene
Cedrene
Scheme 8.15
The formal total synthesis of (
±
)-
α
-and
β
-cedrene.