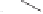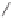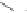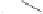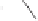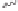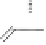Chemistry Reference
In-Depth Information
Tab l e 8 . 1
Pauson-Khand model studies of 2-oxazolone
85
.
TBDPSO
TBDPSO
Et
1. Co
2
(CO)
8
, Et
2
O
2. Pauson-Khand reaction
Et
O
N
N
H
O
H
O
O
O
85
86
T (
◦
C)
Entry
Promoter (eq)
Solvent
Time (h)
Yield (%)
1
heat
MeCN
75 min
75
trace
2
TMANO
•
H
2
O
CH
2
Cl
2
3h
rt
37
3
TMANO
•
H2O
CH
2
C1
2
5.5 h
reflux
55
4
NMO
CH
2
Cl
2
20 h
rt
38
TMANO/4 A MS
5
toluene
12 h
−
10
60
6
i
-PrSMe
C1(CH
2
)
2
C1
45 min
reflux
16
7
CyNH
2
C1(CH
2
)
2
C1
30 min
reflux
11
satisfactory yields and conditions developed by Perez-Castells
51
using TMANO and 4 A
molecular sieves in toluene at a low temperature provided a 60% yield of tricyclic
86
.The
conditions in entries 6 and 7 employing the conditions of Sugihara
52
provided lower yields
of
86
, albeit they are useful in other systems.
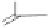
Once effective conditions of the Pauson-Khand reaction were developed for the key
oxazolone intermediates, the focus turned toward the total synthesis of the target molecule.
As shown in Scheme 8.14, a mixture of diastereomers
89
was constructed by a two-step
TBDPSO
TBDPSO
TBDPSO
NaH, DMF, 0 °C, (83%)
1. Co
2
(CO)
8
,Et
2
O
2. TMANO, 4
Å
MS, toluene
-10 °C (51%)
NaHMDS, MeCHO, THF
-78 °C (86%)
OH
H
NH
O
N
N
I
Me
O
O
O
O
87
O
88
89
TBDPSO
Me
Me
OH
TBDPSO
OMOM
1. p-NO
2
C
6
H
4
CO
2
H, PPh
3
, DEAD, benzene, 60 °C
2. Conc. HCl, THF, 60 °C
3. Dess-Martin periodinane, CH
2
Cl
2
,rt(65%)
1. MOMCl, Pr
2
NEt, CH
2
Cl
2
,reflux
2. NaBH
4
,CeCl
3
, MeOH, 0 °C (90%)
O
OH
N
H
N
H
H
H
O
O
O
O
90
91
TBDPSO
Me
Me
O
O
OH
1. K
2
CO
3
,MeOH,rt
2. TBAF, THF, rt (82%)
OR
OH
N
H
H
N
H
O
H
O
O
O
R= p-NO
2
C
6
H
4
CO
93
(±)-8
α
-Hydroxystreptazolone
92
Scheme 8.14
The total synthesis of (
±
)-8
α
-hydroxystreptazolone.