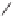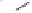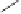Chemistry Reference
In-Depth Information
system which is typically present in the guaianolide skeletal system. Mukai et al. chose
(
)-achelensolide as their target and employed Rh(I)-catalyzed allenic Pauson-Khand re-
actons, as shown in Scheme 8.11.
43
+
Me
MOMO
OPiv
O
10 mol % [RhCl(cod)]
2
50 mol % dppd, 1 atm CO
toluene, reflux, 24 h (96%)
O
OMOM
Me
Me
Me
O
O
OPiv
Me
63
65
64
Me
Me
Me
H
H
H
H
1. AIBN, Bu
3
SnH
benzene, refllux
2. CrO
3
, H
2
SO
4
acetone, H
2
O, rt
O
O
O
O
O
O
O
O
O
H
H
Br
Me
Me
Me
66
21%
68
67
(+)-achalensolide
Scheme 8.11
Total synthesis of (
+
)-achalensolide.
The synthesis began with the known epoxide
63
derived from isoascorbic acid
44
from
which the key allenyne intermediate
64
was synthesized in five steps. Several condi-
tions were explored for the Rh(I)-catalyzed Pauson-Khand cyclization. The first conditions
studied 10 mol% of [RhCl(CO)
2
]
2
in refluxing toluene under an atmosphere of carbon
monoxide to furnish only a 14% yield of bicyclic enone
65
. The yield was increased to
61% when [RhCl(CO)dppp]
2
was employed under the same conditions. Higher presences
of carbon monoxide resulted in intractable mixtures. A third set of conditions were studied
by preparing [
Cl] by
in situ
preparation of 10 mol% of [RhCl(cod)]
2
and
50 mol% 1,3-bis(diphenylphosphino)propane. When this mixture was heated in refluxing
toluene under an atmosphere of carbon monoxide for 24 hours a 96% yield of the bicy-
cle[5.3.0]decadienone
65
was obtained. Attempts to perform the Pauson-Khand reaction
employing the Wilkinson catalyst [(Ph
3
P)RhCl] were unsuccessful.
With ample quantities of
65
in hand, the subsequent steps afforded bromoacetal
66
and
this was followed by radical cyclization after which Jones oxidation gave tricyclic
67
in
15% yield from
65
. The four step synthesis from
65
which remained was carried out to
provide the target
68
(
Rh(CO)(dppp)
2
}
{
+
)-achalensolide. This completed the total synthesis of (
+
)-
68
from
(-)-isoascorbic acid.
8.8 The Total Synthesis of (-)-Alstonerine
The alkaloid (-)-alstonerine
84
possesses structural features such as an indole ring and an
azabridged bicyclic motif and has been the subject of three total syntheses and one formal
synthesis.
45
In addition to the synthetic challenges,
84
may have cytoxic activity against
human lung cancer cell lines.
46
Martin et al. have reported the use of the intramolecular
Pauson-Khand reaction in the total synthesis of indole alkaloids and have recently directed
their focus towards an enantioselective total synthesis of (-)-alstonerine
47
.