Chemistry Reference
In-Depth Information
Kotha,
14
Mehta,
15
and Cook.
16
Recently, a new approach to these molecules has been de-
signed and executed which employed a tandem Pauson-Khand reaction to regiospecifically
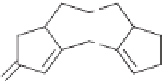
generate the tetracyclic framework of cross linked annulenes
29
and
30
.
17
This work pro-
vided systems required for the construction of
29
and
30
in a higher oxidation state than
those achieved previously.
17d
The required bisalkene
bisalkynes
31
and
33
illustrated in Scheme 8.6 could be accessed
on large scale.
17d
A variety of conditions for the Pauson-Khand reaction were employed
while higher yields were realized with the molybdenum mediated tandem cyclization to
provide tetracyclics
32
and
34
. The process was highly efficient with the generation of six
carbon-carbon bonds in excess of 90% for each bond formed. The tetracylic ring systems
32
and
34
could also be synthesized rapidly on gram-scale; however, Van Ornum recently
showed it was difficult to isolate a tetraene (a dicyclopentadiene) related to
32
.
18
Although
related tetraenes had been observed by
1
HNMR spectroscopy,
18
attempts at isolation had
resulted in polymerization or decomposition. Either the cyclopentadienes were too unstable
or the acetonide began to decompose, consequently, this approach was modified to furnish
intermediates in higher oxidation states using a new approach employing an allenic tandem
Pauson-Khand reaction.
19
−
O
O
1. 67%
2. 74%
3. 82%
H
H
O
O
H
H
O
O
31
32
H
o
O
O
1. 62%
2. 90%
3. 89%
H
H
O
O
H
O
33
34
1. 3eq.Co
2
(CO)
8
,NMO,CH
2
Cl
2
2. 0.2eq.Co
2
(CO)
8
, DME, CO, light (Q-Beam)
3. 2.4eq.Mo(CO)
8
, 10 eq. DMSO, toluene, 100
°C, 4.5 hr
Scheme 8.6
The tandem Pauson-Khand reaction.
It was decided to stabilize the cyclopentadiene substituents with bulky groups, analogous
to the previous approach of Hafner et al.
20
In addition, although the stereocenters in
32
and
34
would eventually be destroyed, it might be difficult to carry the mixture on in succeeding
steps. Moreover, one might choose a precursor in a higher oxidation state closer in energy to
the target 14
cross-linked annulene. This would provide a tetracycle which would require
fewer high energy steps to approach the target than previously envisaged.
17
Based upon
a molybdenum hexacarbonyl mediated allenic Pauson-Khand reaction by Brummond
21
to provide cyclepentenones, a modified approach to provide the key diol is shown in
Scheme 8.7.