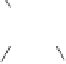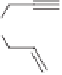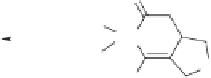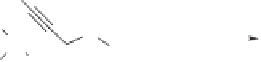Chemistry Reference
In-Depth Information
R
3
R
1
R
3
S
R
1
10 mol% Pd(tmtu)
TsN
TsN
O
Me
2
N
NMe
2
THF, 50
°
C, 24-48 h
CO (Balloon pressure)
R
4
R
2
R
4
R
2
tmtu
43-96% yield
R
1
=H,Ph,alkyl
R
2
=H,CH
2
OBn
R
3
=H,
n
-pentyl
R
4
=H,Et
Scheme 7.41
Pd(tmtu)-catalyzed PKR of
N
-tethered 1,6-enynes.
R
15 mol% [Pd(tmtu)]
R
1-15 mol% LiCl
Z
Z
O
THF, 60
°
C, 48 h
CO (Balloon pressure)
43-91% yield
R=Ar,alkyl
Z = O, C(CO
2
Me)
2
,NTs
Scheme 7.42
Pd-catalyzed PKR with LiCl additive.
In 2009, Wiest/Yang/Wu and co-workers proposed a mechanism for a Pd-catalyzed in-
tramolecular Pauson-Khand-type reaction based on both DFT calculations and experimental
studies.
75
The first step is suggested to involve the
cis
-halometallation of the alkyne moiety,
and is then followed by sequential alkene and carbonyl insertions. The rate-determining step
is an intramolecular C-Cl oxidative addition, in generating Pd(IV) species (Scheme 7.43).
Finally, a reductive elimination as usual gives the desired product Table 7.4.
R
R
Cl
CO
Cl
O
Cl
Pd
Pd
O
L
Cl
L
O
R
Cl
L
R
Pd
O
O
O
Cl
R
O
O
Cl
Cl
O
Cl
Pd
Cl
Pd
L
O
L
O
R
R
Scheme 7.43
Proposed mechanism of Pd-catalyzed 1,6-enynes PKR.