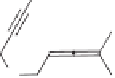Chemistry Reference
In-Depth Information
Ph
O
Ph
9 mol% Ir(phox)SbF
6
DME, 120
°
C, 24 h
CO (2.2 bar)
Z
Z
O
Ph
2
P
N
Ir
*
Up to 97%
ee
98% yield
SbF
6
-
Z = O, NTs, C(CO
2
Me)
2
Ir(phox)SbF
6
Scheme 7.28
Asymmetric Ir(phox)SbF
6
catalyzed PKR of 1,6-enynes.
desired cyclopentadienones were obtained in up to 99% isolated yield. Notably, they also
reported that isocyanides could substitute CO and formed the corresponding iminocy-
clopentadienes by a [Rh(cod)Cl]
2
complex.
Ph
5 mol% Vaska's complex
Ph
EtO
2
C
EtO
2
C
EtO
2
C
EtO
2
C
O
Xylene, 120
°
C, 5 h
CO (1 atm)
Ph
Ph
99% yield
Scheme 7.29
Vaska's complex-catalyzed coupling alkyne-alkyne with CO.
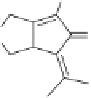
To sum up, the Ir- and Rh-catalyzed Pauson-Khand-type reactions interestingly showed
different regioselectivitives in allenyne cyclization. Allenyne possessing two terminal sub-
stituents on the allene was favoured to be catalyzed by an Ir-complex under a low par-
tial pressure of carbon monoxide. The internal
-bond of the allene moiety was the
major reaction site and the bicyclic cyclopentenone with an alkylidene substituent was
obtained (Scheme 7.30).
56
In contrast, when RhCl(CO)(PPh
3
)
2
was applied, instead of
IrCl(CO)(PPh
3
)
2
as the catalyst under the same reaction conditions, the reaction of external
-bond of allene moiety was mainly dominant. Further to these complementary investiga-
tions, the Shibata group also demonstrated the enantioselective version of this protocol by
using a chiral BINAP ligand instead of triphenylphosphine.
57
R
R
5 mol% IrCl(CO)(PPh
3
)
2
Xylene, 120
°
C
CO (0.2 atm)
Z
O
Z
Favored by
Rh catalyst
Favored by
Ir catalyst
52-91% yield
Scheme 7.30
Regioselectivity of allenyne substrate in PKR governed by Ir-/ Rh-catalyst.