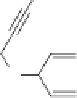Chemistry Reference
In-Depth Information
Jeong applied the iridium system in the desymmetrization of meso-dienynes.
52
A
highly enantio- and diastereoselective Pauson-Khand-type reaction proceeded smoothly
to
afford
vinyl-substituted
bicyclic
cyclopentenones
with
two
stereogenic
centers
(Scheme 7.26).
R
R
R
15 mol% [Ir(cod)Cl]
2
30 mol% (
R
)-BINAP class
Z
O
+
Z
O
Toluene, 130 °C
CO (1 atm)
Z
H
H
Up to 96%
ee
Up to 84%
ee
R = H, Me, Ph
Z = O, NTs
Scheme 7.26
Ir-catalyzed desymmetrization of meso-dienynes.
Shibata further revealed that the chiral Ir-complex derived from (
S
)-tol-BINAP ligand
proved to be an efficient catalyst for decarbonylation of aldehyde and the corresponding
metal carbonyl was utilized for PK cyclization (Scheme 7.27).
51
Though the product yield
was generally moderate, the enantioselectivity was high (85-95%) which exceeded that of
Rh-catalyzed PKR.
16
Independently, the Kwong group also reported a similar protocol to
access a series of optically active bicyclic cyclopentenones by using an (
S
)-BINAP-iridium
complex.
53
R'
5 mol% [Ir(cod)Cl]
2
R'
10 mol% (
S
)-tol-BINAP
Z
R
Z
O
Xylene, 120 °C, 5-24 h
Cinnamylaldehyde (5 eq.)
*
R
85-92%
ee
30-66% yield
R = H, Me
R' = aryl, alkyl
Z = O, NTs, C(COOEt)
2
Scheme 7.27
Asymmetric Ir-catalyzed PKR with aldehyde as CO source.
Recently, Pfaltz reported a chiral cationic Ir(phox) complex for application in an in-
tramolecular asymmetric Pauson-Khand-type reaction (Scheme 7.28).
54
Under optimized
reaction conditions, the desired products were obtained with high yields and enantioselec-
tivities. Lowering of the catalyst loading was possible for
O
-tethered 1,6-enyne substrates
(91% ee with 1 mol% of Ir catalyst). The influences of CO pressure and counter anion
were also studied. The CO pressure significantly affected the reaction rate rather than enan-
tioselectivity. A higher product yield was obtained under a higher CO pressure. Moreover,
the study of counter anion indicated that hexafluoroantimonate was found to be the most
effective counter pair for the complex.
Apart from the carbonylative alkene-alkyne coupling, Shibata realized an alkyne-alkyne
coupling with carbon monoxide effectively (Scheme 7.29).
55
They applied Vaska's com-
plex IrCl(CO)(PPh
3
)
2
to perform the intramolecular [2
+
2
+
1] cycloaddition of diyne. The