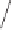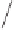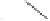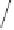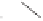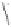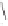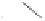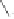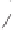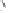Chemistry Reference
In-Depth Information
and in a drive to enhance the selectivity of the alkene incorporation, Krafft turned to the
use of heteroatoms tethered to the olefin in attempts to control annulation regiochemistry.
Using homallylic (or bishomoallylic) sulfides and amines, as functional units capable of
acting as soft donor ligands, both the efficiency and regioselectivity of these PK processes
were shown to be appreciable, and certainly when compared to the previously established
and normally much less effective intermolecular reactions (Scheme 1.8).
15
These outcomes
are believed to result from the tethered heteroatom acting as a ligand for cobalt along the
reaction pathway, in turn acting to restrict the conformational flexibility of the complexed
alkene, and leading to the more favourable formation of the 5-substituted products.
O
O
SMe
SMe
Tol., 90 °C
30 h
Ph
Ph
+
+
Ph
H
Co
2
(CO)
6
1
61%
18 : 1
SMe
NMe
2
O
O
Tol., 90 °C
30 h
77%
Ph
Ph
+
+
Ph
H
Co
2
(CO)
6
NMe
2
NMe
2
1
20 : 1
Scheme 1.8
Following on from this pioneering work by Krafft and co-workers in the area of directed
intermolecular PK reactions, studies within our own laboratory have led to the development
of an analogous system with allylphosphonates. Optimised conditions with phenylacetylene
complex
1
and diethyl allylphosphonate
11
led to the formation of cyclopentenones
12
in
high yield and good levels of regioselectivity.
16
In due course, it was demonstrated that
an extended class of alkenes possessing a tethered phosphonate ester unit could also be
employed within P-K processes, again with good levels of olefin regiocontrol (Scheme 1.9),
to deliver an additional class of phosphorus-containing cyclopentenones of further synthetic
potential.
17
O
O
O
O
P(OEt)
2
P(OEt)
2
MeCN/DCM,
reflux, 18 h
Ph
Ph
+
+
Ph
H
Co
2
(CO)
6
O
1
82%
P(OEt)
2
11 : 1
1 1
1 2 a
1 2 b
O
P(OEt)
2
O
O
O
MeCN,
reflux, 15 min
p-MeC
6
H
4
P(OEt)
2
+
p-MeC
6
H
4
H
Co
2
(CO)
6
O
O
O
71%
+
O
O
p-MeC
6
H
4
O
P(OEt)
2
12 : 1
O
Scheme 1.9