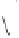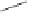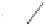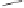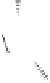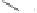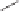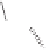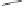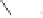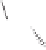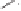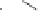Chemistry Reference
In-Depth Information
O
O
H
H
1) MeOH, h
benzophenone
ν
TIPS-Cl
TMS
2) TBAF, THF
DMF, Imid
OH
H
H
78%
95%
(-)-3i
50
O
H
O
OH
AlEtCl
2
, MA
DIBALH
CH
2
Cl
2
, 55 °C
Et
2
O, -78 °C
OTIPS
H
OTIPS
OTIPS
85%
70%, 12: 1
52
53
51
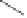
Scheme 6.31
Preparation of the homochiral allyl alcohol
53
from the PK cycloadduct (-)-
3i
.
The nucleobase was then introduced via palladium-catalyzed allylic substitution onto the
methylcarbonate of alcohol
53
(which gave better yields than the corresponding acetate).
When 6-chloro-2-purinamine was used as nucleophile, and sodium hydride as base, the
nucleoside
54
was obtained in good yield and in excellent optical purity (
99% ee, as
determined by HPLC). Compound
54
was ultimately transformed into the final products
Abacavir and Carbovir by standard chemistries (Scheme 6.32).
O
N
NH
N
N
NH
2
Cl
OH
N
N
OH
1) Cl-COOEt
(-)-Carbovir
Pyr, CH
2
Cl
2
(94 %)
N
N
NH
2
2) 6-chloro-2-purinamine
NaH, Pd(PPh
3
)
4
,
DMF, (67%; d.r. 4:1)
OTIPS
HN
OTIPS
N
N
53
54
N
N
NH
2
OH
(-)-Abacavir
Scheme 6.32
Synthesis of Carbovir and Abacavir from the homochiral allyl alcohol
53
.