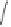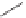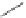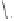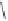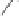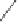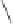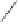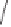Chemistry Reference
In-Depth Information
unsaturated aldehyde
46
, afforded, in a one-pot reaction, the exocyclic enone with complete
stereoselectivity. The retro-Diels-Alder reaction, using microwave-assisted chemistry and a
modified version of the conditions developed by Grieco, provided the desired phytoprostane
in good yield and excellent enantiomeric purity.
Phytoprostane dPPJ
1
type II was synthesized with the same approach. In this case, since
the
side chain holds the ester functionality, the conjugate addition had to be performed
using a silyl protected alcohol as a precursor of the ester. After the cuprate addition, 2-
pentenal was added to the reactionmixture to afford the exocyclic enone
48
in excellent yield
(Scheme 6.30). The silyl ether was converted into themethyl ester via high-yielding standard
protocols: acid deprotection of the silyl ether; oxidation to the aldehyde with Dess-Martin
periodinane (DMP); oxidation to the acid with sodium chlorite; and finally, esterification
with trimethylsilyl diazomethane. The retro-Diels-Alder reaction (again, using themodified
version of Grieco's conditions), afforded phytoprostane dPPJ
1
-II methyl ester.
1)
TBSO
CuLi
O
O
8
H
1) AcOH/H
2
O/THF
2) DMP
H
Et
2
O/pentane
TMS
O
3) NaClO
2
,
t
-BuOH, H
2
O
4) Me
3
SiCHN
2
, PhH/MeOH
2)
OTBS
H
8
H
H
(+)-
3i
(99% ee)
48
O
O
H
MeAlCl
2
/MA
COOMe
DCM, microwave
COOMe
(9
S
,13E,15E)-dPPJ
1
-II methyl ester
94% ee
H
49
Scheme 6.30
Synthesis of dPPJ
1
-II methyl ester from enantiopure (
+
)-
3i
.
Carbanucleosides are an important class of nucleoside analogs, some of which, whether
natural (e.g. Aristeromycin and Neplanocin) or synthetic (e.g. Carbovir and Abacavir), ex-
hibit anti-tumor and anti-viral activities. In fact, Abacavir has been approved and launched
for the treatment of HIV. The Riera and Verdaguer group reasoned that the highly function-
alized five-membered rings featured in these compounds made them excellent targets for
synthesis via the PKR.
49
They envisaged that for reaction of the PK cycloadduct
3i
as chiral
starting material, the key reactions would comprise introduction of the nucleobase and ad-
dition of the hydroxymethyl side chain. Although many d
1
-synthons can be added to
3i
with
high yields and complete stereoselectivity, the simplest transformation is photochemical
conjugate addition of methanol, a procedure originally developed by Fraser-Reid.
50
Thus,
photochemical conjugate addition of methanol to
3i
, using one equivalent of benzophenone
as a triplet sensitizer,
51
proceeded cleanly. Subsequent cleavage of the silyl group by TBAF
afforded the bicyclic compound hydroxymethylcyclopentenone
50
in good yield. Protection
of the alcohol as the silyl ether, followed by retro-Diels-Alder, gave the key intermediate
52
. This protected cyclopentenone was diastereoselectively reduced with DIBALH to give
the allyl alcohol
53
(Scheme 6.31).