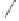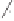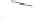Chemistry Reference
In-Depth Information
*
P
S
BH
3
*
*
CO
CO
P
S
S
P
OC
CO
OC
OC
DABCO
CO
CO
Co
Co
Co
Co
Co
Co
OC
CO
OC
CO
OC
CO
Toluene
65 °C
, 5 h
Toluene
75 °C, 16 h
TMS
TMS
TMS
dr 3:1
1i
31ia'
31ia
Mother
liquor
O
Crystallization
Ph
2
P
S
O
H
OC
CO
Co
Co
10 eq.
96% yield
96% ee
(99% upon rec.)
OC
CO
TMS
TMS
NMO 6 eq.
CH
2
Cl
2
, 40 °C
H
31ia
(+)-3i
66% yield, 6 g
(2 cycles)
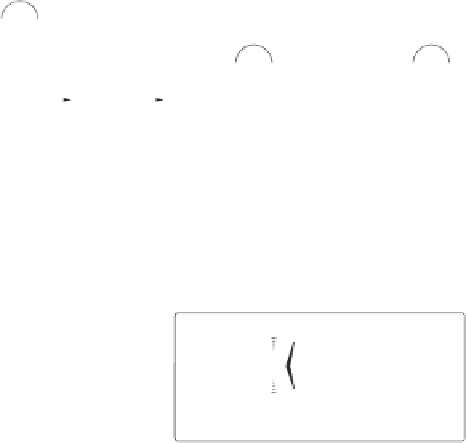
Scheme 6.26
Formation of the Pauson-Khand adduct
3i
using PuPHOS. Preparation of di-
astereomerically pure complex
3ia
via dynamic resolution.
equilibration/crystallization. Starting from (
R
)-
tert
-butylsulfinamide for the preparation of
ligand
35b
, the levorotatory cycloadduct
3i
was obtained in 98% ee (again, the ee can be
further increased by recrystallization).
O
1)
S
PPh
2
PMB
t-Bu
N
PMB
O
OC
CO
O
N
P
h
Ph
35b
H
OC
CO
t-Bu
S
P
Co
Co
Toluene, 65
°
C
OC
OC
CO
CO
TMS
Co
Co
NMO, CH
2
Cl
2
, 40
°
C
OC
CO
2) 70
°
C, 20 h
3) Crystallization
TMS
H
TMS
84% yield
98% ee (99% upon rec.)
1i
67%
37ib
(-)-3i
Scheme 6.27
Multi-gram enantioselective synthesis of the PK cycloadduct
3i
using the PNSO
ligand
35b
.
Cycloadduct
3i
can be considered a chiral synthon of cyclopentadienone. The enone
functionality enables a wide range of chemistries; in particular, it is an excellent sub-
strate for conjugate additions. Additionally, the trimethylsilyl group can be easily removed
and the second enone can be unmasked (via retro-Diels-Alder reaction) (Scheme 6.28).
Thus, organocuprate addition, followed by simple treatment with TBAF, gave bicyclic
ketones that, after retro-Diels-Alder reaction, provided enantioenriched 4-substituted cy-
clopentenones.
46
However, access to
-disubstituted cyclopentenones has proved to be
problematic, due to lack of reactivity and to formation of polyalkylation side-products dur-
ing introduction of the
,
-side chain. To date, the best reported methodology for overcoming