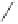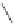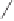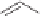Chemistry Reference
In-Depth Information
which have proven to be valuable in this transformation (Scheme 6.13, Table 6.1). Since
then, the BINAP-Co combination has become a workbench system for studying catalytic
PKRs (
vide infra
), although proficient applications have been restricted to the intramolecular
version. Importantly, these examples all require high catalyst loadings (20 mol%). A related
methodology that employs a
C
2
-symmetric bidentate phosphite was described in 2002
by Buchwald.
23
The combination of the BINOL-derived bis(phosphite)
18
and catalytic
amounts of dicobalt octacarbonyl effected intramolecular PKR of diverse enyne substrates
with disparate degrees of enantiocontrol. The most significant improvement offered by this
methodology is that it entails lower catalyst loadings (6 mol%). Interestingly, Buchwald
discovered a case of matched/mis-matched chirality upon incorporating BINOL fragments
of different configurations into diastereomers of
18
(Scheme 6.13).
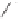
O
O
PA r
2
PA r
2
R
PPh
2
Me
PPh
2
PPh
2
P
O
O
O
R
PPh
2
Me
O
P
O
O
(
S
)-BINAP Ar = Ph
(
S
)-TolBINAP Ar =
p
-Tol
(
R
)-BIPHEMP R = Me,
(
R
)-MeOBIPHEMP R = OMe
(
R
,
R
)-DIOP
18
Scheme 6.13
Ligands used in cobalt-catalyzed intramolecular asymmetric PKRs (see Table
6.1).
At first glance (Table 6.1), the relative success of
C
2
-symmetric ligands in these transfor-
mations could be rationalized in terms of the general ability of highly symmetric ligands to
reduce the number of diastereomeric transition states through symmetry elements. However,
numerous kinetic and theoretical studies on this system do not corroborate this premise.
In 1999 Laschat demonstrated that although ligand exchange reactions of dicobalt-alkyne
clusters could produce bridged complexes with BINAP and other achiral bis(phosphines),
24
these clusters did not engage in any PK chemistry under standard conditions. Conversely, in
an extended series of kinetic studies,
25
Gibson showed that chelated coordination of BINAP
(which would yield
C
1
-symmetric dicobalt-alkyne clusters downstream to PK adducts) is
responsible for the catalytic activity of this catalyst-ligand combination in the cyclization
of enynes. This thorough investigation included identification (by
31
P NMR and X-ray
crystallography) of the chelated BINAP-Co
2
(CO)
6
cluster as a PK precatalyst; this precat-
alyst gave the bicyclic enone in 88% ee
22
(Scheme 6.14). A recent theoretical study by
Maseras, Pericas et al. rationalized the outcome of asymmetric intramolecular PKRs using
the cobalt-BINAP system.
26
In terms of cobalt-catalyzed reactions, the BINAP system is the only one that delivers
useful levels of asymmetric induction. However—and very much like every other asymmet-
ric intramolecular PKR—chemistry of the BINAP system is strongly limited in substrate
scope: it only works for some 1,6-enynes. Further substitution at either the alkene or the