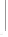Chemistry Reference
In-Depth Information
OSi
i
Pr
3
OSi
i
Pr
3
O
O
O
NMe
2
O
NMe
2
Me
Me
OsO
4,
TMEDA,
CH
2
Cl
2,
-78 °C
O
O
S
S
N
Os
87
O
N
O
81%
Me
Me
85f
To l u e n e ,
reflux,
72%
OSi
i
Pr
3
OH
O
O
Me
Me
O
2M aq HCl, rt
(-)-pentenomycin I
(> 99% ee)
HO
O
88
N
Os
HO
O
76%
N
O
Me
Me
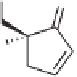
Scheme 5.58
5.4 Chiral Reagents for the Kinetic Resolution of PK Cycloadducts
If the PKR cannot be performed in an enantioselective fashion (either by using a chiral
auxiliary or a chiral metal ligand), a kinetic resolution of the racemic PK cycloadduct can
also be envisaged. This strategy was pioneered by Hua, 25 years ago, in his remarkable
asymmetric total synthesis of (
)-pentalenene (Scheme 5.59).
59
The racemic intramolecular
PK adduct
rac
-
89
, obtained in 58% yield from the corresponding enyne precursor, was
treated with 0.5molar equivs of the lithium derivative of (
S
)-allyl
p
-tolyl sulfoxide in THF
at
+
78
◦
C gave the adduct
90
, derived from (
R
)-
89
(80% yield), and the unreacted (
S
)
enantiomer of
89
(45% yield, 82% ee). This compound was subsequently converted into
(
−
)-pentalenene in an eight-step sequence.
Several years later, Schmalz and co-workers applied the kinetic resolution of racemic
PK cycloadducts in a synthesis of novel carbocyclic nucleoside analogs.
60
These authors
investigated the utility of the asymmetric Corey-Bakshi-Shibata reduction of ketones
61
for
this purpose (Table 5.13). When the racemic
exo
-allyloxyenones
91a
-
c
, obtained in good
yields by an intramolecular PKR, were treated with catecholborane (0.80mol equivs) in
the presence of the (
R
)-Me-CBS oxazaborolidine catalyst, the unreacted ketones
91
were
recovered in very high enantiomeric purity. The fast-reacting enantiomers of
91
gave rise
+
H
R
Me
Me
CO
CO
N
Co
Co
OC
CO
S
O
Figure 5.2