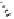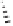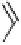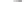Chemistry Reference
In-Depth Information
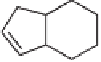
Table 5.11
O
H
O
O
O
H
Xc
H
Xc
Me
O
Me
OC
OC
Exo
-
71a
,
76a
O
Mo
Co(CO)
3
Xc
Toluene, 90 °C
H
H
Me
Endo
-
83a
,
b
Me
81a
,
b
82a
,
b
H
Xc
O
Exo
-
70c
Complex
Xc-
Product
Yield (%)
Dr
O
83a
24
4:1
81a
ON
71a
12
> 20:1
70c
62
5:1
Ph
O
92
1:9
83a
81b
ON
71a
4
n.d.
3
n.d.
70c
Ph
Me
Me
83b
36
> 20:1
82a
,
b
76a
18
> 20:1
N
O
2
S
The reaction takes place in moderate to good yields, with total regioselectivity with
respect to the alkyne component, and with excellent diastereoselectivity. With internal
alkynes (
Cf.
2-butyne) the sulfoxide
84
did not react under standard conditions, and
high pressures (10 Kbar) were necessary to achieve a 33% yield of the adducts mix-
ture (11.5:1 dr), at room temperature. The authors showed that the reaction took place
with retention of the stereochemistry at the sulfur, and that the pairs of diastereomers
85
and
86
differed only in the configuration at the carbon atom. The major diastere-