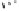Chemistry Reference
In-Depth Information
Compound
69a
gave rise to two diastereomeric complexes
81a
and
81b
, differing in the
chirality of the heterobimetallic cluster, that were separated by column chromatography.
The sultam derivative
74c
also afforded two diastereomeric complexes that could not be
separated. When each of the diastereomers of
81
was reacted with norbornadiene under
thermal conditions, a mixture of the
endo
-adduct
83a
(only the 2-carbamoyl regioisomer)
with the two regioisomers
70c
and
71a
(both as diastereomer mixtures) was isolated. On
the other hand, the diastereomeric mixture of
82
gave rise to the
endo
-adduct
83b
and to the
exo
-2-carbamoyl regioisomer
76a
(Table 5.11). It is worth noting that the two diastereomers
of
81
display opposite diastereoselectivity, so that the chirality of the C
2
CoMo core appears
to control the diastereoselectivity of the formation of the
endo
-fused adducts. In the case
of
82
, both adducts
endo
-
83b
and
exo
-
76a
were obtained as single diastereomers (by
13
C-
NMR), which tends to indicate that only one isomer of the heterobimetallic complex
82
had
reacted. These results are similar to those discussed above (Scheme 5.49, Section
5.3.4
),
reported shortly afterwards (2003) by Shen and Hsung.
50
It is worth noting that the dimolybdenum complexes of
N
-(2-alkynoyl)oxazolidinones
did not react with norbornadiene, suggesting that the cobalt atom is the active one in the
intermolecular PKR of the heterobimetallic complexes. Examination of molecular models
of these heterobimetallic complexes showed that the presence of an oxazolidinone or
sultam moiety could force the bulky cyclopentadienyl ligand to occupy an eaquatorial (i.e.,
cis to the Mo-Co bond) coordination site. This would then distort the geometry of the
ligands around the cobalt and destabilize the coordination of the olefin from the
exo
face
(Scheme 5.57).
O
O
Xc
Me
CO
CO
Xc
Me
OC
OC
OC
Mo
Co
Mo
Co
OC
CO
CO
Scheme 5.57
5.3.6 Alkenyl Sulfoxides
In contrast to their successful application in intramolecular PKRs,
28, 30, 31
chiral
O
-alkyl
enol ethers (and chiral alkoxyallenes) give poor results in the intermolecular version of the
process.
57
This, together with the fact that in general only strained olefins give good yields
in the intermolecular PKR, delayed the development of asymmetric approaches based on
chiral auxiliaries bonded to alkenes. It was not until 2003 that Carretero and co-workers
demonstrated that vinyl sulfoxides, in particular the potentially cobalt-coordinating
2-(
N
,
N
-dimethylamino)phenyl vinyl sulfoxide (
R
)-
84
(easily available in
99% ee from
commercial reagents) reacts with a variety of alkyne-dicobalt hexacarbonyl complexes
with high levels of regio- and stereoselectivity (Table 5.12).
58