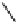Chemistry Reference
In-Depth Information
Concordant with the author's expectations, when a solution of
52a
in hexane was allowed
to stand at room temperature under a current of dry nitrogen for CO evacuation, it underwent
partial transformation to the more polar, chelated dicobalt pentacarbonyl complex
53a
(Scheme 5.39).
40
A maximum 85:15 ratio of
53a
and
52a
was achieved by heating the
solution of the parent hexacarbonyl complex
52a
at 50
◦
C for 1 h with CO removal. When
this mixture of complexes was cooled back to room temperature under CO atmosphere,
the hexacarbonyl complex
52a
was regenerated. Finally, treating
52a
with an excess of
N
-methylmorpholine-
N
-oxide (NMO) in dichloromethane under nitrogen led to the total
formation of the chelated species
53a
.Bothby
1
HNMR and by TLC analysis,
53a
appeared
to be a single diastereomer, probably as a result of thermodynamic control. DFT calculations
suggest that the methylthio group coordinates at the axial site of the pro-(
R
) cobalt atom.
43
A similar behavior was observed for complexes
52b
and
52c
.
43
Me
Me
Me
Me
-CO (
Δ
)
O
O
H
H
+CO (rt)
S
Me
S
Me
Co
Co(CO)
3
(OC)
3
Co
Co(CO)
3
NMO excess
OC
CO
53a
52a
Scheme 5.39
20
◦
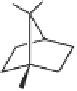
Cwith an excess of a strained olefin
(norbornene, norbornadiene, or bicyclo[3.2.0]hept-6-ene), the corresponding PK adducts
54
,
55
and
56
were obtained in good yields and with remarkable diastereoselectivities (up
to 24:1 dr in the case of
55
; Table 5.7). On the other hand, reaction with a less reactive
olefin such as cyclopentene, that required heating of
52a
at 60
◦
C for 20 h in a sealed tube,
afforded the cycloadduct in low yield (14%) and with no selectivity (1:1 dr).
43
In the case
of
55
, the major diastereomer could be separated after careful column chromatography.
The absolute configuration of the major isomer of
55
was unambiguously ascertained by
X-ray diffraction analysis of a dihydrogenated derivative, as well as by chemical correlation
with the known cyclopentenone (-)-
57
(Scheme 5.40), obtained by a sequence of reactions
similar to that depicted in Scheme 5.36 above, involving stereoselective cuprate addition
from the
exo
face, reductive cleavage of the auxiliary with samarium diiodide and retro-
Diels-Alder cycloaddition.
35
This stereochemical outcome (coincident to that afforded by (1
R
,
2S
)-2-
phenylcyclohexanol)
35
could be rationalized with the aid of DFT calculations, that sug-
gested a reaction path involving coordination of norbornadiene (from the
exo
face) to the
site occupied by the methylthio group in the pro-(
R
) cobalt (Scheme 5.41).
43
The application of asymmetric intermolecular PKRs of chiral alkoxyacetylenes to the
enantioselective synthesis of natural products was subsequently exemplified by Greene and
co-workers in a formal total synthesis of the fungal metabolite (
When the preformed complex
53a
was treated at
−
+
)-brefeldin A (Scheme
5.42).
44