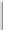Chemistry Reference
In-Depth Information
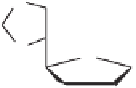
Tab l e 5 . 6
O
H
*RO
H
*RO
+
(OC)
3
Co
Co(CO)
3
Isooctane, reflux,
18 h
H
46a
-
e
Product
R*O-
Yield (%)
Dr
O
46a
52
2:1
Ph
46b
25
> 10:1
O
O
Me
46c
46
2:1
Me
O
O
O
O
O
Me
Me
Me
Me
Me
Me
Me
O
O
46d
30
3:1
Me
Me
Me
Me
O
Me
46e
46
1.6:1
O
O
Me
classical thermal conditions, to afford the 2-alkoxy-substituted cyclopentenones
45a
-
f
and
46a
-
e
, respectively. In accordance with the general behavior of intermolecular PKRs of
terminal acetylenes,
10, 36
not only was the regioisomer in which the alkyne substituent is
vicinal to the carbonyl group predominantly formed, but the
exo
-annulated diastereomers
of
45a
-
f
were the only ones found.
While the use of
trans
-2-(9-phenanthryl)cyclohexanol led to the formation of adducts
45b
and
46b
with complete diastereoselectivity, the difficult preparation of this alcohol in