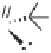Chemistry Reference
In-Depth Information
Me
Me
Me
O
S
Me
Me
Me
O
Me
Me
Me
O
O
n
-BuLi, THF, -78
°
C;
S
S
+
(EtO)
2
P
81%,
56:44
H
H
H
CHO
Cis-
35a
Tr an s -
35a
Co
2
(CO)
8,
CH
2
Cl
2,
rt;
MeCN, reflux, 15 min
Me
Me
Me
Me
Me
Me
O
O
S
S
H
H
O
36a
(44%)
O
37a
Scheme 5.30
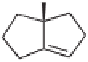
C
-
Re
face, since the opposite C
-
Si
face is blocked by the
tert
-butyl group. The resulting
cobaltabicyclooctane intermediate then evolves to the observed adduct.
32b
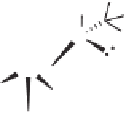
Me
Me
Me
O
Me
Me
Me
H
Me
Me
Me
O
O
S
H
H
OC
S
C
CO
S
X
OC
X
Co
O
Co
X
H
CO
H
Co(CO)
3
(OC)
3
Co
H
H
Scheme 5.31
As the last step of these asymmetric PKRs stereocontrolled by sulfoxides, the reductive
cleavage of the
tert
-butyl sulfinyl group can be efficiently carried out by treatment with
activated zinc, giving the highly enantiopure (
96% ee) adducts
38
(Scheme 5.32). Thus,
the
tert
-butylsulfinyl group acts as a removable, efficient stereochemical controller in the
intramolecular PKRs of 1-sulfinylenynes, although
strictu sensu
it can not be considered
as a chiral auxiliary, since it cannot be recovered after its removal.
5.2.6 Asymmetric Intramolecular PKRs Mediated by Chiral Auxiliaries
Located in the Enyne Tether
This approach to asymmetric PKRs has remained largely unexplored. In 1994, Salaun, de
Meijere, and co-workers reported
33
that the cobalt-mediated bicyclizations of 1,6-enynes
39-
-
c
, with a methylenecyclopropane terminator and a chiral acetal moiety adjacent to