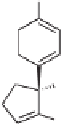Chemistry Reference
In-Depth Information
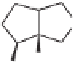
a reaction sequence involving base-promoted isomerization of the propargyl ether
25
and
regio- and diastereoselective (9:1 (
E
)/(
Z
) mixture) addition of propargyl thiol to the resulting
homochiral allene
26
(Scheme 5.26).
KO
t
Bu,
t
BuOH,
reflux, 2 h
HC
CCH
2
SH
Ph
Ph
Ph
27
(77%)
H
O
O
O
80%
HBF
4
cat.
CH
2
Cl
2
, -78 ºC
to rt, 12 h
S
C
25
26
H
H
H
H
Scheme 5.26
Exposure of
27
to dicobalt octacarbonyl in isooctane, followed by heating at 90
◦
Cfor
2.5 h, produced a
ca.
12:1 mixture of diastereomeric adducts
28
from which the major one
could be isolated in 33% yield by column chromatography (Scheme 5.27). No products
Me
p
-tolyllithium,
CuI, Et
2
O,
-
10 to 0 °C, 10 min
;
Co
2
(CO)
8
27
S
S
29
(41%)
O
O
rt, 12 h
Isooctane, rt, 0.5 h;
reflux, 2.5 h
H
H
*RO
*RO
28
(Major isomer, 33%)
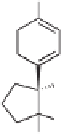
Ni-Raney,
EtOH, reflux
Me
Me
Me
SmI
2,
THF-MeOH,
-70 °C, 5 mi
n
1) LDA, PhSeBr,
T
HF, -78 to 0
°
C
Me
Me
Me
29
(41%)
O
O
O
Me
2) H
2
O
2,
aq AcOH,
rt, 1 h
Me
Me
31
(77%)
H
H
*RO
30
(90%)
Me
2
Zn, cat. Ni(acac)
2,
Et
2
O, rt, 15 h
Me
Me
(+)-
β
-cuparenone
O
Me
Me
Scheme 5.27