Chemistry Reference
In-Depth Information
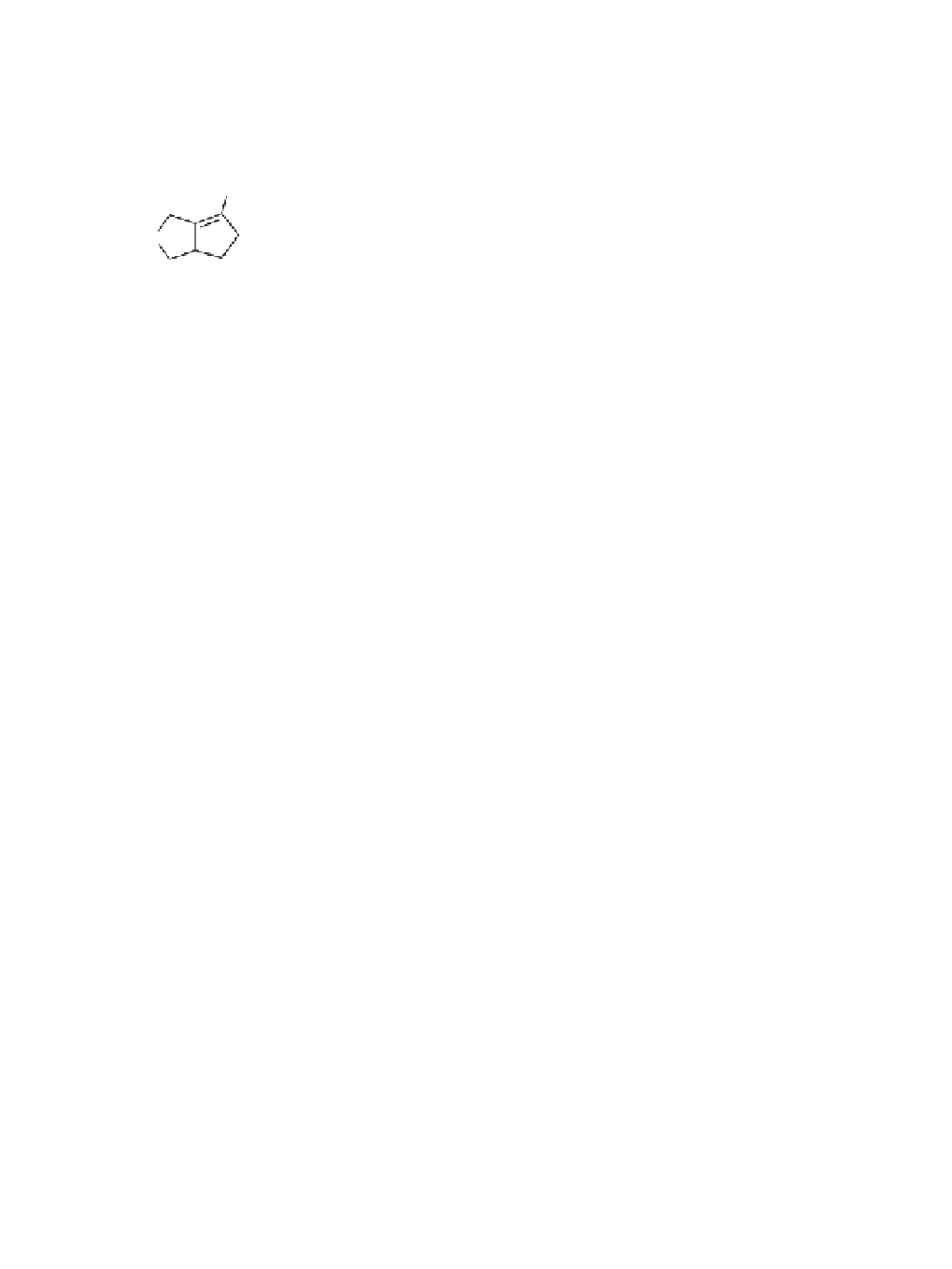
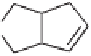
Treatment of enones
7a
and
7b
(major diastereomers) with a catalytic amount of hy-
drochloric acid in methanol led, with complete regioselectivity and in excellent yield, to
both enantiomers of the
-methoxyenone
8
, with a nearly quantitative recovery of the
alcohols
4a
and
4b
, respectively (Schemes 5.13 and 5.14). This allowed an unambiguous
assignment to be made of the absolute configuration (5
S
) of the major diastereomer of
7a
.
Me
Me
O*R
O
H
Cat. HCl, MeOH
OH
O
Me
+
O
OMe
O
O
Me
Me
Me
H
H
7a
(Major diastereomer)
(+)-
8
(94%)
4a
(96%)
Scheme 5.13
Me
O*R
O
Me
Me
Me
Me
H
Cat. HCl, MeOH
O
+
O
OMe
O
O
OH
Me
H
H
7b
(Major diastereomer)
(-)-
8
(83%)
4b
(94%)
Scheme 5.14
As happened with
trans
-2-phenylcyclohexanol,
16, 17
molecular mechanics calculations
offered a ready explanation for the stereochemical outcome of the reaction. Thus, the
cyclization of
6b
was proposed to occur from a conformation such as that shown in
Scheme 5.15, in which the pro-(
R
) cobalt tricarbonyl group is shielded by the neopentyloxy
substituent of the chiral auxiliary. Then, interaction of the
Si
face of the olefin with the
pro-(
S
) cobalt leads to a (5
R
) configuration in
7b
.
Me
Me
Me
Me
Me
Me
Me
Me
Me
Me
Me
Me
Me
Me
O
O
O
O
O
Me
O
Co(CO)
3
Co(CO)
3
Me
Me
Me
CO
CO
O
O
Co
O
Co
(CO)
3
H
H
O
(Major)
Scheme 5.15