Biomedical Engineering Reference
In-Depth Information
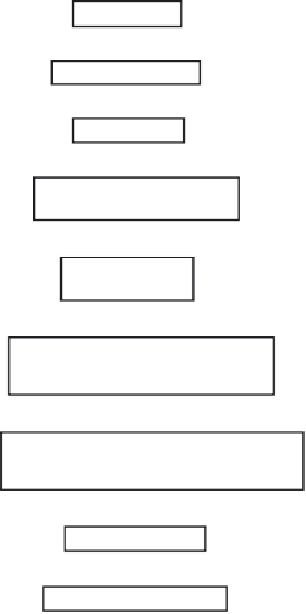
Drug Discovery
Initial characterization
Pre-clinical trials
Regulatory approval sought to
commence trials in humans
Clinical trials
(Phases I, II & III)
Submission of marketing/manufacturing
authorization applications to
regulatory authorities
Regulatory authorities review information and
grant (or refuse) marketing/manufacturing
licences
Product goes on sale
Post-marketing surveillance
Figure 4.1
An overview of the life history of a successful drug. Patenting of the product is usually also
undertaken, often during the initial stages of clinical trial work
Regulatory involvement does not end even at this point. Post-marketing surveillance is gener-
ally undertaken, with the company being obliged to report any subsequent drug-induced side ef-
fects/adverse reactions. The regulatory authority will also inspect the manufacturing facility on a
regular basis in order to ensure that satisfactory manufacturing standards are maintained.
4.2 Discovery of biopharmaceuticals
The discovery of virtually all the biopharmaceuticals discussed in this text was a knowledge-
based one. Continuing advances in the molecular sciences have deepened our understanding of
the molecular mechanisms that underline health and disease. An understanding at the molecular
level of how the body functions in health and of the deviations that characterize the development
of a disease often renders obvious potential strategies likely to cure/control that disease. Simple
examples illustrating this include the use of insulin to treat diabetes and the use of GH to treat
certain forms of dwarfi sm (Chapter 11). The underlining causes of these types of disease are rela-
tively straightforward, in that they are essentially promoted by the defi ciency/absence of a single


Search WWH ::

Custom Search